Abstract
Inactive fusion variants of the CRISPR-Cas9 system are increasingly being used as standard methodology to study transcription regulation. Their ability to readily manipulate the native genomic loci is particularly advantageous. In this work, we serendipitously uncover the key cytokine IL6 as an off-target of the activating derivative of CRISPR (CRISPRa) while studying RP11-326A19.4, a novel long-non coding RNA (lncRNA). Increasing RP11-326A19.4 expression in HEK293T cells via CRISPRa-mediated activation of its promoter region induced genome-wide transcriptional changes, including upregulation of IL6, an important cytokine. IL6 was increased in response to distinct sgRNA targeting the RP11-326A19.4 promoter region, suggesting specificity. Loss of the cognate sgRNA recognition sites failed to abolish CRISPRa mediated activation of IL6 however, pointing to off-target effects. Bioinformatic approaches did not reveal predicted off-target binding sites. Off-target activation of IL6 was sustained and involved low level activation of known IL6 regulators. Increased IL6 remained sensitive to further activation by TNFα, consistent with the existence of independent mechanisms. This study provides experimental evidence that CRISPRa has discrete, unpredictable off-targeting limitations that must be considered when using this emerging technology.
Introduction
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) related technologies are revolutionizing gene editing. Not only do CRISPR-associated (Cas) proteins allow for relatively facile genomic editing, inactive variants are continuously being created to study gene function [1,2]. Specificity of Cas9 has been extensively studied [3]. Optimal cleavage by spCas9 requires anRNA co-factor, either a two-part guide RNA component (native context) or in a more artificial and practical setting, a single-guide RNA (sgRNA) consisting of a 20-mer spacer sequence that confers cleavage specificity and a longer generic scaffold. In addition, the cognate DNA sequence must be followed by a NGG protospacer adjacent motif (PAM). This requirement allows, in theory, spCas9 to cut at a unique site of the human genome. In practice however, cleavage can occur at other sites [3,4]. Furthermore, variants harnessing the DNA homing properties of Cas proteins, and in particular Cas protein 9 (Cas9), are continuously being engineered to be used as research tools. The resulting fusion proteins are typically comprised of a deactivated Cas9 (dCas9) that retains its DNA binding properties, fused to various regulatory domains that can influence genomic regulation, including transcriptional activation and repression (CRISPRa and CRISPRi, respectively). Compared to wild-type Cas9, dCas9 fusions need only to bind DNA in the proper orientation to impart de novo function. Current evidence indicates that each sgRNA has the potential to direct Cas9 or dCas9 to tens to thousands of sites [5,6]. While this represents an upper limit and de facto binding events are likely not as pervasive due to nucleosome interference [7], the genome contains abundant potential off-target binding sites. Whether these binding events persist long enough to have functional outcomes is unclear.
Driven by increasingly sensitive genome-wide expression analyses, a new level of regulatory control has emerged over the last decade, as it was realized that a significant proportion of the genome which was heretofore believed to be transcriptionally silent was rather expressing long transcripts. Although they lack salient protein coding potential, these long non-coding RNAs (lncRNA) form a class of RNAs in many respects similar to traditional mRNA: lncRNA are relatively long, contain polyA tails and undergo alternative splicing, although splicing of lncRNA is typically more extensive [8]. In addition conservation tends to be lower than for protein coding genes [9,10]. Some but not all lncRNA appear to be functional [11], which may reflect our inability to properly test for biological significance [12]. To account for their lack of conservation, it has been proposed that the very act of transcription at lncRNA loci, rather than the transcript per se, determines function [13]. With these features in mind, the advent of CRISPRa methodology seemed particularly suited to tackle lncRNA function as targeting CRISPRa/i upstream of lncRNA genes in principle 1- permits transcriptional activation (or repression) and 2- induces changes in the native transcript levels, including all forms of spliced variants, whose ratio may be important for function.
IL6 is a broadly expressed cytokine with wide ranging impacts on multiple biological systems. Key implications of IL6 in cancer, inflammation, coronary artery disease (IL6R), hematopoiesis, and metabolic processes have been reported [14–17]. Using different binding modalities in specific contexts, IL6 provides either pro- or anti-inflammatory cues. A considerable body of literature dealing with regulation of IL6 has shown it to be under extensive transcriptional and translational control. Post-transcriptionally, IL6 is a relatively unstable transcript, regulated by several microRNA and protein regulators that interact with the 3’ UTRs of the mature transcript [15].
Here we demonstrate that targeting CRISPRa to distinct regions of a novel lncRNA promoter region leads to IL6 upregulation, largely via off-target effects. The mechanisms underpinning the activation implicate increased transcription via a low level activation of established IL6 inducers. These findings add a note of caution regarding the reliability of current CRISPRa technology.
Results
Validation of a long non-coding RNA situated proximal to a region associated with coronary artery disease
While investigating the role of a GWAS-identified locus for Coronary Artery Disease (CAD) at15q26 near MFGE8 [18], we identified a region encoding putative, uncharacterized transcripts (RP11-326A19.4/TCONS_00023492) immediately adjacent to the risk region (Fig 1A). The proximity of the transcripts to the CAD region hinted at a possible link to atherosclerosis, thus warranting further investigation of the gene locus. Examination of public data indicated that the corresponding gene is predicted to span 42 kbp and encodes alternatively spliced transcripts characterized by some exon conservation but low coding potential, typical of a long non-coding RNA (S1 Fig). We confirmed the existence of annotated exons by qRT-PCR using pairs of primers spanning predicted intronic sequences in HEK293T cells, consistent with the presence of at least two distinct transcripts (referred to below as RP11 for simplicity) (Fig 1B).
Fig 1. Validation of RP11-326A19.4/TCONS forms in HEK293T.
A, Manhattan plot of the 15q.26 CAD risk locus. Top, local Manhattan plot Data was drawn with LocusZoom using CAD association data from Nikpay et al [34]. Bottom corresponding genes and transcripts from GENECODE (green and blue) and Human Body Map (brown), visualized via the UCSC browser data, to scale with the Manhattan plot. B, splice form analysis of RP11-326A19.4. RNA was isolated from HEK293T and subjected to qRT-PCR analyses with primer sets targeted against various exons of the predicted transcripts. Top, schematic of primers used for PCR and alternating exon organization. Black bars represent exons shared by both spliced forms while orange bars represent additional exonic sequences unique to the TCONS_00023493 form. Size of predicted introns are indicated on top. Bottom, agarose gel of products. Primer pairs used are indicated above the gel. Predicted sizes of PCR fragments (in bp) are indicated under the gel and blue arrows point to the predicted migration position. In addition primers targeting exon 1 and 2 resulted in the presence of an additional band, longer by about 100 bp, consistent with the presence of an an alternative exonic sequence, situated between predicted exon 1 and exon 2.
Identification of a promoter region governing RP11 expression
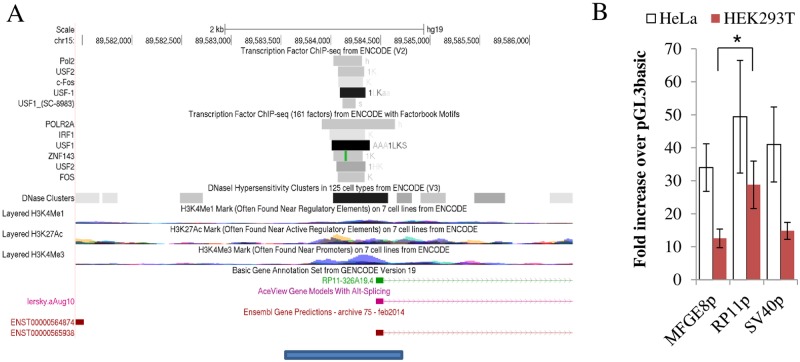
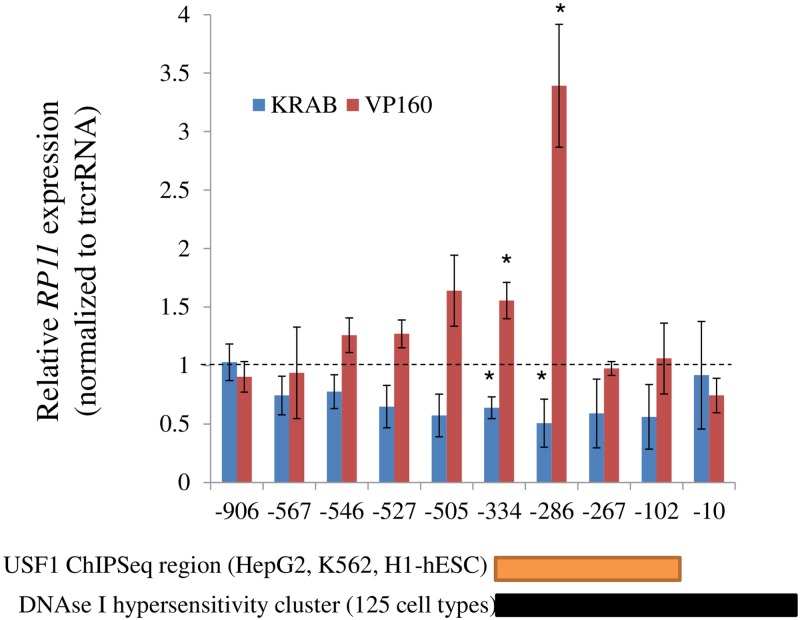
The multiple possible splice variants suggested a complex transcriptional output that may be important for function. In this regard, the ability of the dCas9 derivatives to modulate both transcript abundance and complexity via endogenous gene regulation, seemed appropriate for the study of RP11. First, this approach was contingent on the identification of the promoter region of RP11. Exploiting annotated chromatin marks, proximity, ChiP and DNAse sensitivity, a ~ 1.5 kb region that included the putative exon 1 of RP11 was identified (Fig 2A). In several cell types, the region is predicted to interact with general transcription factors (USF2, USF1, FOS) and core transcription machinery (POLR2A). To examine its ability to operate as a promoter, the corresponding region was subcloned upstream of a luciferase reporter and introduced in HEK293T and HeLa cells. Presence of the putative promoter conferred robust luciferase activity, reaching levels on par with promoters derived from SV40 and MFGE8, a gene flanking RP11 whose transcript is abundant and widely expressed (GTex) in both HeLa cells and HEK293T cells (Fig 2B). Functional validation of the putative promoter was performed using the CRISPRa and CRISPRi systems. Constructs expressing dCas9 fused to either the VP160 activator or the KRAB repressor were introduced into HEK293T cells, in conjunction with sgRNA expressing plasmids to target CRISPRa/i to the RP11 promoter region. Transfection of CRISPRa and CRISPRi plasmids resulted in either activation or repression, respectively, of RP11 expression that reflected the predicted cognate sgRNA binding site: the impact was maximal in the middle of the region and attenuated on either side (Fig 3); interestingly the region of maximal activation was central to reported DNase I hypersensitivity and ChIP Seq region. DNA accessibility seemed insufficient for activation as regions located closer to the transcription start site did not support activation, reflecting the presence of other constraints (e.g. CRISPRa position relative to the transcription machinery, orientation, lack of binding etc…).
Fig 2. Identification and characterization of putative RP11 promoter.
A, Identification of putative promoter region. Promoter region (blue bar) used for reporter assay is shown under a 5 Kb UCSC browser snapshot of chIP-seq results, DNAse I hypersensitiviy and epigenetic marks from ENCODE. Putative promoter includes ChiPseq peaks as well as a DNAse rich region, as indicated. B, Proximal region upstream of RP11 operates as a strong promoter in isolation. Activity of three promoter regions assessed by reporter assays, expressed relative to the promoterless control (pGL3basic). SV40, a strong promoter as well as the putative promoter region proximal to the MFGE8 transcriptional start site are included for comparison. Data represent the average of 4–6 experiments for HeLa and 3 experiments for HEK293T (± 95% C.I.). All promoter constructs increased luciferase activity significantly (p = 0.05–0.001) relative to the pGL3 basic control. RP11p had levels significantly higher than MFGE8p (p <0.05) only in HEK239T.
Fig 3. Validation of the promoter region by CRISPRi and CRISPRa.
A, HEK293T cells were transfected with sgRNA expressing plasmids in conjunction with either Cas9 fused to a KRAB repressor (CRISPRi) or a VP160 activator (CRISPRa). Expression levels of RP11 were measured 48 h later. Numbers on the x-axis refer to the predicted binding region of the sgRNA, relative to the transcription start site of RP11-326A19.4, set to 1. Expression values were normalized first to peptidylprolyl isomerase A (PPIA) values and then divided by the corresponding values from within experiment control transfected with trcrRNA and CRISPRa expressing plasmids. ChIPseq and DNAse I hypersensitivity sites are from ENCODE. Results represent the average of 3 biological replicates (± S.D.).
Altered RP11 transcription does not affect local gene expression
The impact on neighboring genes was investigated under the assumption that the RP11 locus affects local transcription, in line with proposed models of lncRNA actions. As illustrated above (Fig 1A), the RP11 locus is flanked by 2 protein coding genes MFGE8 and ABHD2 and further away by HAPLN3. The effect of RP11 activation and repression on these as well as 2 control housekeeper genes, including SRP14 located distantly on the same chromosome, was assessed by qPCR analysis. Transfection of SG-286 along with CRISPRa or CRISPRi had no significant impact on either local or housekeeper gene levels (SRP14 and GAPDH) (S2 Fig). Thus RP11 levels and/or transcription activity do not appear to regulate regional transcriptional output.
Genome-wide transcriptome interrogation of changes associated with CRISPRa of RP11 reveals an upregulation bias
To identify putative RP11 targets that might help elucidate its function, the transcriptome-wide impact of increased RP11 transcription was assessed. We reasoned that effects secondary to RP11 activation, be they at the transcriptional or post-transcriptional levels, should be mirrored by changes in the transcriptional fingerprint of the cell. Importantly, introduction of CRISPRa elicits a restrained RP11 response which should minimize non-specific effect effects associated with typical transduction type experiments. Transcriptome profiles of HEK293T cells transfected with CRISPRa alongside SG-286, which led to the strongest RP11 increase (3–4 X), or a control tracrRNA construct, were compared using gene expression arrays (Clariom D). The analysis confirmed a 4.4 increase in RP11. Furthermore, focusing on nominally significant (p <0.05, > 2-fold linear change) and validated transcripts (no AceView transcripts), the analysis revealed 220 genes, of which only 39 were downregulated (S1 Table). The departure from a more evenly distributed repressive/stimulating profiles expected a priori, suggested that a large proportion, perhaps most, of the changes resulted from off-target binding and activation by CRISPRa. Alternatively, these results may point to a role of RP11 in promoting transcription. Looking for possible genomic binding motif that could account for this, genome-wide interrogation of differentially expressed transcripts by Overrepresentation Enrichment Analysis (ORA) via WebGestalt indicated no FDR-significant enrichment motifs within 4 kb of the transcription start sites of impacted genes, although a few motifs reached nominal significance (Table 1). Thus changes in expression cannot be ascribed to the presence of a particular subset of transcription factor binding motifs within the genome.
Table 1. Over Representation analysis of transcription factor targets.
| Motif | Size | Expect | Ratio | P Value | FDR |
|---|---|---|---|---|---|
| TGGNNNNNNKCCAR_UNKNOWN | 409 | 2.576 | 3.8821 | 0.000232 | 0.082281 |
| IK3_01 | 213 | 1.3415 | 5.218 | 0.000377 | 0.082281 |
| GTGGGTGK_UNKNOWN | 285 | 1.795 | 4.4569 | 0.000412 | 0.082281 |
| NKX61_01 | 226 | 1.4234 | 4.9179 | 0.000538 | 0.082281 |
Over Representation Analysis of transcription factor targets via WebGestalt. The analysis looks within a gene list for a particular pattern that is enriched in a subset of gene. In the current analysis, promoter sequences of the nominally affected genes and the entire gene list are first screened for the presence of putative as well as validated transcription factor binding sites. Binding sites that are enriched in the normally affected list (i.e. more present than expected by chance) may indicate shared underlying effectors. RNAs isolated from HEK293T cells transfected with dCas9-VP160 alongside either trcrRNA or SG-286 were subjected to transcription array analyses. Transcripts showing more than 2-fold and nominally significant (p<0.05) changes were mapped to 135 unique Entrezgene IDs; more speculative genes (Pseudogenes, lncRNA and Aceview transcripts) were excluded from the analysis. Of the 135 IDs, 76 were annotated to the functional categories and were present in the reference list. The reference list was mapped to 21491 Entrezgene IDs of which 12067 were annotated to functional categories. Multiple comparison correction was performed by False Discovery Rate according to Benjamini Hochberg and only the FDR values lower than 0.1 are shown; FDR significance threshold is 5% (0.05). Size, number of genes in the reference list; Expect, expected representation; Ratio, ratio of Obtained/Expected; P value, nominal p value; FDR, False Discovery Rate (q value).
IL6 transcript and protein levels are increased in response to CRISPRa targeting of RP11
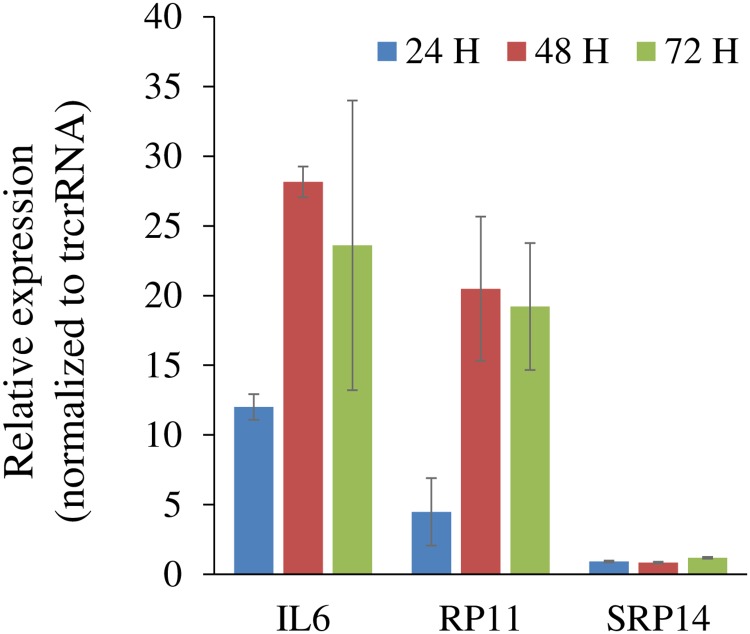
Among the list of nominally affected genes (S1 Table), we noted the presence of 2 cytokines that hinted to a possible implication of RP11 in the regulation of the immune response: IL6 and IL36B. Both were confirmed to be upregulated by qPCR (Fig 4A for IL6 (10.3-fold ± 3.2) and 3.7-fold (± 1.3) over control (n = 3, p = 0.07) for IL36B). As IL36B expression was very low in HEK293T (Cp ~ 36), we chose to focus on IL6 whose transcript was readily detectable, albeit at low abundance by qRT-PCR (Cp~ 30). Comparable results were obtained with 2 non-overlapping pair of IL6 qPCR primers, ensuring detection specificity (See S1 Supplementary Materials for primer information). Moreover, increased transcript levels were accompanied by higher IL6 protein concentration in the media, as assessed by enzyme-linked immunosorbent assay (ELISA) (Fig 4B). In contrast to CRISPRa, CRISPRi (KRAB), which in combination with SG-286 inhibited RP11 expression by ~30%, had no noticeable impact on IL6 (Fig 4A). The relatively small impact on RP11 is likely due to the design of SG-286, which is optimized for activation (upstream of the transcription start site) rather than inhibition (internal to the transcript). These results suggest that RP11 upregulation, but likely not its downregulation, may regulate IL6 expression.
Fig 4. CRISPRa affects IL6 expression in HEK239T.
A, regulation of RP11 transcription by repressive (KRAB) or activating (VP160) Cas9 derivatives. Cells were transfected with an activator or a repressor, in the presence of SG-286 or trcrRNA alone. Set used was independent from Fig 5. Data from 3 independent experiments (except for [IL6/ RP11] + KRAB, 5 experiments). B, CRISPRa activation increases secreted IL6 levels. IL6 protein concentration was measured in the media of HEK293T cells transfected for 48 h with CRISPRa and either SG-286 or trcrRNA alone.
IL6 is increased in response to CRISPRa using sgRNA designed against distinct regions of the RP11 promoter
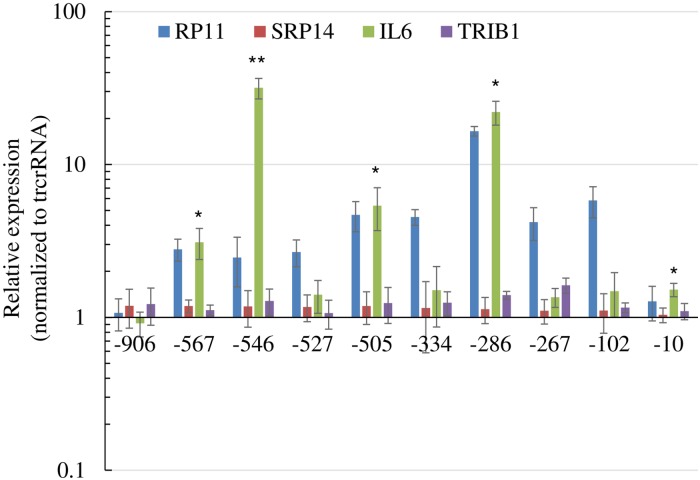
CRISPR/Cas9 related technologies suffer from specificity limitations and increased IL6 associated with RP11 upregulation could be the result of off-target CRISPRa binding. To more specifically link transcription activation of RP11 by CRISPRa to IL6, levels of IL6 were examined in response to multiple sequences covering the promoter region. Moreover, a distinct activator (VPR vs VP160) was used, which resulted in more potent and pervasive activation of RP11 (Fig 5). Significantly, increased IL6 was observed with half of the sgRNAs. By comparison, two other control transcripts (TRIB1 and SRP14) were only modestly affected, although some reached nominal statistical significance (TRIB1, SG-286 and SG-267).
Fig 5. Increased expression of IL6 by CRISPRa directed toward several regions of RP11 promoter.
HEK293T transfected with dCas9-VPR and sgRNA constructs were analyzed. Values for RP11 represent the average from 3 distinct primer combinations spanning exons 1–2, 2–3, and 3–4. Two specificity controls (SRP14 and TRIB1) are included. Expression values were normalized first to PPIA and then to a within experiment control transfected with dCas9-VPR and the empty trcrRNA expressing plasmid. Results represent the average of 3 biological replicates (± S.D.). For simplicity only statistically significant tests for IL6 are shown (*: p<0.05; ** p<0.01).
The RP11 promoter region is rich in IL6 activation sequences
Despite some inconsistencies (e.g. SG-546), which were inferred to stem from off-target binding to the IL6 gene, these experiments argued for a functional relationship between the RP11 locus and IL6. Presumably, this could reflect a general propensity of IL6 to respond to activation by CRISPRa. To examine this possibility, the MFGE8 promoter situated 130 kb upstream of the RP11 gene was in turn targeted by CRISPRa. We used an approach similar to that of the RP11 locus, whereby multiple sgRNA sequences were used to capture any possible off-target effects. For a subset of sgRNA, targeting dCas9 to MFGE8 resulted in increased MFGE8 expression. However, increased transcript output was not accompanied by increased IL6 levels (S3 Fig). This underscores the particular functional relationship of IL6 with the RP11 promoter region.
IL6 induction by CRISPRa does not require the RP11 locus
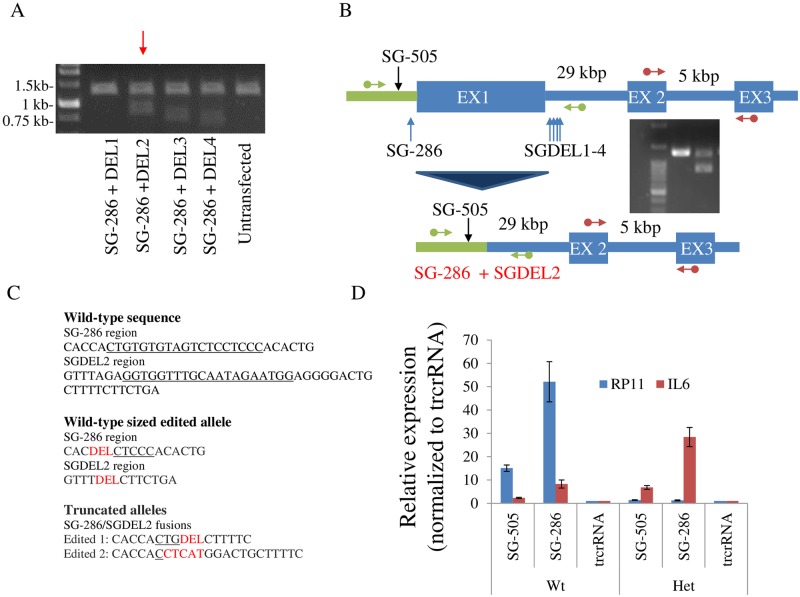
To assess the role of RP11 in controlling IL6, exon 1 of RP11 along with its proximal regulatory region was deleted using CRISPR/Cas9 by simultaneously targeting the SG-286 and a sequence within intron 1. Unfortunately, the process yielded only compound heterozygotes (Fig 6). Sequencing of the Wt sized allele revealed that both sgRNA cognate sites employed for deletion were extensively edited. Unexpectedly, despite retention of an otherwise intact RP11 allele, RP11 became resistant to CRISPRa induction by SG-505, whose binding is predicted to occur upstream of the deletion. Importantly, cells retained the ability to induce IL6 in response to CRISPRa. These results indicated that the RP11 transcript was dispensable for IL6 induction, but left open the possibility that interchromosomal contacts could play a role. Alternatively, this could indicate that activation was through off-target binding by CRISPRa. Specificity was first tested for SG-286, whose cognate sites were absent in the compound heterozygotes. Surprisingly introduction of dCas9-VPR alongside SG-286 still augmented IL6 levels, thus demonstrating off-target effects, at least for that sgRNA. In light of these findings, the specificity of SG-505 was tested by generating a SG-505 binding site knock-out (using Cas9 and SG-505), which again revealed that RP11 but not IL6 upregulation was abrogated by the deletion (S4 Fig). Together these findings indicate that RP11 and IL6 are functionally uncoupled and that impacts on the latter are mediated by off-target mechanisms.
Fig 6. CRISPR/Cas9 editing of RP11 abrogates RP11 induction but does not prevent IL6 induction by CRISPRa.
A, removal of a region spanning 418 bp by CRISPR/Cas9. Expected size of native fragment is 1.3 kbp. Using 2 distinct sgRNA, deletion by Cas9 results in appearance of a novel band of reduced size (~ 0.9 kbp for SG-286/DEL2 combination). B, schematic of the deletions. Green arrows indicate position of PCR primers used to validate gene editing. Red arrows indicate qRT-PCR primers (recognizing exon 2 and 3) used in D. Drawing not to scale. C, Sanger sequencing results of the edited alleles. For the Wt sized allele, a single deletion pattern was observed wherein both sgRNA recognition sites were deleted extensively. As for the shorter allele species, two distinct sequences, all with edited recognition sites, were observed. The cognate sgRNA sequence, or residual sequences thereof, are underlined. Deletions (DEL) and insertions are indicated in red. D, Impact of deletion on compound heterozygotes cells targeted by CRISPR/Cas9 as in A, using SGDEL2 and SG-286 sgRNAs. A mixture of 3 heterozygote clones were compared with 3 Wild-type clones (treated with CRISPR/Cas9 only) Data represent the average of 3 experiments, performed over 3 passages (± S.D.).
Off-target prediction algorithms fail to identify IL6 as a direct target of CRISPRa
However surprising for 2 distinct sgRNA sequences, these observations suggested that increases in IL6 and RP11 share similar underlying processes, i.e. sgRNA steers dCas9-VPR to the IL6 locus. Indeed we observed a slow accumulation of IL6 which paralleled RP11’s and which contrasted with classical IL6 induction mechanisms which involve acute responses (Fig 7). Searches using commonly used off-target algorithms trained on CRISPR sgRNAs failed to identify SG-286 or SG-505 sites matching IL6 [19,20]. Unfortunately, these algorithms are optimized for prediction of relative (off-target) cleavage rather than binding. Similarly, tools incorporating CRISPRa predictions allow only for a few mismatches from the optimal 20 mer consensus and failed to identify any off-target [21,22]. CROP-IT which allows for more extensive misalignments and binding (up to 10 mispaired nucleotides) identifies ~110,000 genome-wide binding sites for SG-505 and SG-286 [23]. However, none were in the vicinity of IL6 (S2 Table). If bulges in RNA and/or DNA are allowed [24], the number of binding sites increases dramatically (S3 Table). For instance, allowing a 5 nucleotide mismatch and 1 RNA bulge on a 20-mer sequence allows for ~600,000 potential targets or 1 binding site every ~5.5 kb bases in a 3.3 Mb human diploid genome, or on average ~ 5 binding sites for a “typical” 28 kb gene [25]. Thus, current prediction tools are unfortunately of limited utility to recognize in situ off-target dCas9 binding sites.
Fig 7. Upregulations of RP11 and IL6 occur in parallel.
Time course of SG-286 activation in HEK293T. Cells were transfected with dCas9-VPR and SG-286 or trcrRNA for the indicated times. Indicated RNAs were quantified by qRT-, normalized to PPIA values and then divided by the trcrRNA/dCas9-VPR. Data represent the average of 3 experiments (± S.D.).
Activation of IL6 by CRISPRa involves multiple regulatory pathways
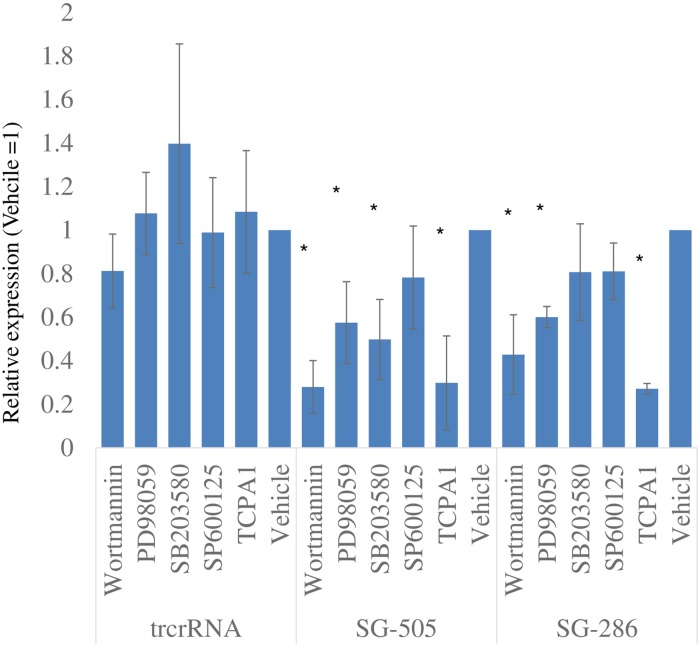
Having demonstrated induction of IL6 by this experimental approach, we turned our attention to the underlying regulation, anticipating that identification of mechanisms at play may shed light on possible upstream regulators and IL6 regulation in general. Physiological IL6 expression is controlled by several upstream kinases including MAP kinases, PI3K and IKK. The contribution of these regulators to CRISPRa-mediated induction was tested first by targeted inhibitions in HEK293T cells. Using two distinct sgRNAs, all drugs reduced IL6 expression relative to the corresponding vehicle-treated CRISPRa, although the impact of TCPA-1 (IKK), Wortmannin (PI3K) and PD98059 (MAPKK) were more pronounced and reached statistical significance (Fig 8 and S5 Fig for SRP14 control). Interestingly SB203580 (p38 MAPK inhibitor) affected induction by one sgRNA but not the other, hinting that the underlying mechanisms differ in part. By contrast IL6 levels in the control CRISPRa/ trcrRNA samples remained unaffected by the treatments, indicating that these kinases play no role in supporting basal IL6 expression. These findings appear consistent with the activation by CRISPRa involving established IL6 regulators.
Fig 8. Kinase inhibitors interfere with CRISPRa activation of IL6.
HEK293T cells were transfected for 24 h with dCas9-VPR together with the indicated sgRNA constructs, in the presence of kinase inhibitors. Cells were then harvested and IL6, SRP14 and PPIA levels were quantified. Values for IL6 (relative to PPIA) are shown; SRP14 quantifications are shown in S4 Fig. Values are normalized to the corresponding vehicle values to emphasize the impact of the drugs on each CRISPRa transfection and facilitate inter-construct comparisons. Data represent the average of 3 experiments (± S.D.).
CRISPRa does not induce measurable activation of IL6 master regulators
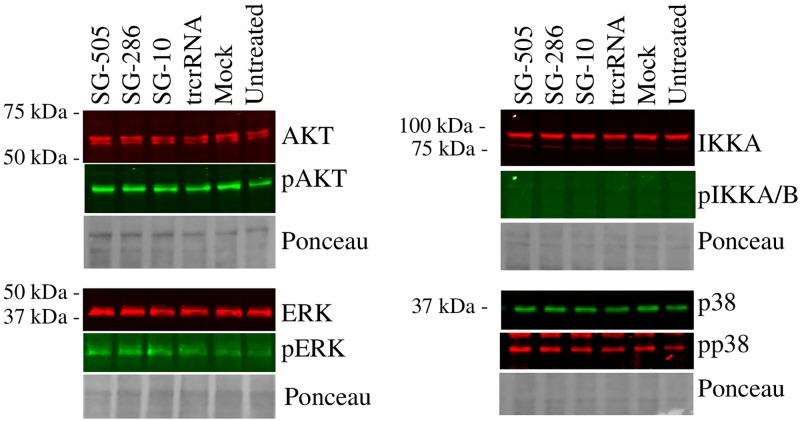
Activation status of these pathways was tested next. As a proxy for activation, phosphorylation levels of ERK (downstream target of the MAPKK axis), IKK, AKT (a downstream target of PI3K) and p38 were examined in response to CRISPRa. Of note, these effectors typically exhibit rapid and transient kinetics that differ from our slower transfection based approach. Still, we reasoned, based on the inhibitor studies, that the steady accumulation of IL6 resulting from the introduction of CRISPRa may reflect enduring activation of these effectors. Introduction of CRISPRa was accompanied by marginal phosphorylation changes, none of which were directionally consistent with the IL6 activation profile (SG-286 >SG-505>SG-10) (Fig 9, S6 and S7 Figs). Thus, increased IL6 is not accompanied by a measurable impact on its predicted regulators. Together with the inhibitor findings, these results point to the activation of established IL6 regulatory pathways, albeit to an extent that is not detectable by our methods. Alternatively, the contribution of distinct effectors sharing sensitivities to the inhibitors cannot be ruled out.
Fig 9. Activation by CRISPRa occurs in the absence of detectable changes in known IL6 regulators.
HEK293T cells were transfected with dCas9-VPR and the indicated SG constructs for 24 h, lysed and analyzed by Western blotting. Quantification is presented in S6 Fig. PhosphoIKKA/B signal was too low to be confidently identified and quantified; a positive control for phosphoIKKA/B (M1 polarization of THP-1 cells) is shown in S7 Fig.
Regulation of IL6 by CRISPRa in a physiological context
We hypothesized that CRISPRa, by artificially inducing IL6 expression via a low grade response may interact, either positively or negatively, with the normal inflammatory response machinery and IL6 responsiveness. IL6 is a well-characterized target of NFKB, a prototypical inflammatory response transcription factor [15]. Upon activation, NFKB dissociates from its dedicated repressor, migrates to the nucleus and binds to specific response elements present in the promoters of a number of genes, including IL6. TNFα is a major proatherogenic regulator and a classic activator of NFKB via the ubiquitously expressed TNFR1receptor 1 (TNFR1) [26]. Thus the response of IL6 to TNFα was examined in the context of CRISPRa activation, anticipating that CRISPRa might impact TNFα activation of IL6. Unfortunately, HEK293T cells were found unsuitable since IL6 levels therein did not respond to TNFα treatment (2 h, 30 ng/ml; 1.2-fold (± 0.24) over vehicle (n = 2). Rather, the impact of CRISPRa on RP11 and IL6 was examined in a different cell type, HuH-7, a hepatoma cell line widely used to study hepatocyte function. Inflammatory components, including IL6, have been shown to play crucial roles in normal and pathological liver functions [27]. As in HEK293T, SG-286 and SG-505 increased RP11, although the fold-increase was more dramatic, possibly due to lower basal expression of RP11 in those cells (S8 Fig). HuH-7 cells were also responsive to TNFα, at least acutely: addition of TNFα for 2 h resulted in increased IL-6 in naïve or mock transfected cells, although statistical significance was only reached for mock (Lipof3000) transfected cells (Fig 10). By contrast, longer treatments had no noticeable impact on IL6. Introduction of dCas9-VPR/SG-286 was sufficient to increase IL6 to levels on par with those reached with TNFα treatment alone. Combining dCas9-VPR/SG-286 with TNFα conferred an additional increase, albeit without noticeable synergy. These results clearly demonstrate that CRISPRa does not interfere with normal TNFα signaling, insofar as IL6 is concerned, but rather suggest that both contribute, independently, to IL6 upregulation.
Fig 10. Distinct effects of TNFα and CRISPRa on the stimulation of IL6 expression.
HuH-7 cells were transfected with dCas9-VPR and the indicated SG constructs for 48 h. TNFα (30 ng/ml) was included for the last 24 h (blue bars) or 2 h (red bars) of transfection, as indicated. Levels of IL6 and PPIA were then measured by qRT-PCR. Pertinent statistical test (1-way ANOVA) results are shown (*, p <0.05; ***, p<0.001). Data represent the average of 3 biological replicates.
Discussion
In a landmark study, systematic dissection of 12 lncRNA loci demonstrated that for all loci investigated, transcriptional activity per se rather than nature of the transcript affects neighboring protein coding genes [28]. CRISPRa/i approaches offer the ability to regulate in situ transcription with unparalleled ease and thus facilitate research on lncRNA. Using CRISPRa/i, we demonstrated that expression of neighboring genes was resistant to RP11-326A19.4 perturbation. Rather a long distance impact on several transcripts, including IL6, was observed. We chose to focus on IL6, based on its known importance in human coronary artery disease. Importantly, validation studies indicated that increased IL6 was the result of off-target activity by CRISPRa.
The observation that 4 out of 10 sgRNA targeting RP11 lead to increased IL6 is intriguing and could be construed to indicate specificity. To our surprise we found that when tested via binding site abrogation, 2 different sgRNA were non-specific, i.e. altered IL6 via other means. The mechanism(s) remain unclear. In contrast to IL6, the same sgRNAs had little or no effect on controls (SRP14 and TRIBI). To control for the promoter region of RP11, 10 other sequences designed to target the MFGE8 promoter region had no or very modest effects on IL6. One possible explanation may be that the RP11 promoter region is rich in sequences that are linked to IL6 upregulation. Although sequence homology searches (BLAST) revealed no salient homology between the IL6 gene and sgRNA used, the proclivity of dCas9 to bind promiscuously leaves open the possibility that it binds to IL6 regulatory regions. In view of the current bioinformatic limitations, teasing out CRISPRa target(s) mediating IL6 upregulation will require genome-wide interrogation of in situ binding sites, followed by their functional validations.
While the events surrounding CRISPRa binding are unclear, our study demonstrates that IL6 upregulation involves traditional IL6 master regulators. This conclusion is based on inhibitor evidence, which blocked IL6 upregulation. However, as phosphorylation remained below detection, increased IL6 could be not be linked to the activation status of the regulators. This could simply reflect the transient nature of the activation which might be reversed at the time of analysis. However we note that activation continues beyond this time point, which seems inconsistent with that interpretation. The progressive, long-term accumulation of IL6 versus the more conventional transient response typically studied, points to an alternative explanation. We speculate that these regulators are activated minimally, yet sufficiently to induce a slow and continuous rise in IL6. This accumulation of IL6 most likely involves both increased output as well as stabilization at the mRNA, both of which are known to control IL6 and immunokine levels in general [29].
dCas9 derivatives are increasingly being leveraged to interrogate biological mechanisms. For instance to understand and manipulate enhancer function, complementary dCas9 variants can be targeted to different regions of the genome thereby forcing their juxtaposition [30]. Correct interpretation of the generated datasets relies on unmitigated binding localization which, as shown here, this technology does not allow. More significantly, therapeutic interventions involving activation of transcription suppressed genes, great care should be exercised given the significant possibility of off-target interactions.
The off-target effects reported here are for Staphylococcus pyogenes Cas9 derivatives, arguably the most commonly used form, largely for historical reasons. Other Cas variants have different cutting specificities but their applicability to multiplexed applications, where binding rather than cutting is the parameter of interest, remains to be as thoroughly investigated as spCas9. In order to obtain robust and truly useful tools, the dCas methodologies must be further improved. Indeed as little as a 5 mer + PAM sequence might be sufficient to confer binding to d(sp)Cas9 in mouse embryonic stem cells [5]. This will require binding optimization, including greater specificity and affinity. The use of shorter oligonucleotides was shown to improve cleavage specificity somewhat but the suitability of this approach to minimize off-target binding is doubtful [31]. Wild-type enzymes have evolved for both binding and cutting, and considerable room exists for molecular engineering approaches to favor binding specificity, likely at the expense of cutting. For instance, Cas9 derived from Neisseria meningitis is a recently described compact Cas9 that exhibits higher cutting fidelity and would be of potential interest in this regard [32]. By contrast, Cas9 from Staphylococcus aureus has been recently shown to be undergo faster turnover which may translate into weaker substrate affinity and mitigate its ability to be effective as CRISPRa/i [33].
This study includes an early investigation of RP11-326A19.4 function which future work will aim to enrich by identifying bona fide effectors. Activating the RP11 locus via CRISPRa/SG-286 led to an upregulation bias, hinting that a substantial subset of the affected genes may in fact stem from off-target binding. To aid with on-target identification, expression changes induced by other RP11 targeting sgRNA will be compared and contrasted [11]. The observation that IL6 was independently upregulated with two sgRNA targeting RP11, reinforces the need for multiple additional sgRNA. Lastly, orthogonal interventions such as antisense oligonucleotides or RP11 transduction are currently underway to examine the role of the transcript proper.
Materials and methods
Tissue culture and drug treatments
Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing either 4.5 g/l glucose (HEK293T, HeLa) or 1 g/l (HuH-7). HEK293T and HeLa were obtained from the ATCC and HuH-7 were purchased from Japanese Collection of Research Bioresources Cell Bank (JCRB Cell Bank). Unless mentioned otherwise all transfections were performed for 48 h using Lipofectamine 3000 (Thermofisher) at ratios of 3:2:1 (Lipof 3000: P3000: DNA). For inhibitor studies, the following drugs were used (working concentration): PD98059 (20 μM), SB203580 (5 μM), SP600125 (1 μM) and Wortmannin (1 μM) were from Cell Signaling Technology. TPCA-1 (20 μM) was from Sigma. Transfections were performed for 24 h in the presence of the inhibitors or vehicle (0.1% DMSO); inhibitors were added 30 min prior to transfection.
Transcription array analysis
Transcriptome profiling on SG-286/CRISPRa transfected cells was performed at the Centre for Applied Genomics (The Hospital for Sick Children, ON, Canada). Purified RNA, was converted to cRNAs and hybridized to Human Clariom D arrays. Results were analyzed by using the Expression and Transcriptome Analysis Consoles (Applied Biosystems); the underlying expression data set is accessible as GSE132451 at the Gene Expression Omnibus (GEO) repository.
Luciferase assays
HEK-293T were seeded in 24 well plates and transfected 48 h later with 0.5 μg DNA using Lipofectamine 3000; pRSV40 (2% of total plasmid) was included as internal control. Cells were transfected for 24 h, lysed and processed for luminescence analysis, measuring firefly and renilla signals in a GLOMAX plate reader.
ELISA
Media from HEK293T cells transfected with for 48 hours with CRISPRa (VPR) alongside either SG-286 or trcrRNA constructs, were recovered. Following a 2 min centrifugation at 2000 x g, the cleared supernatants were frozen at -20 C until further analysis. Media were then thawed and assayed for IL6 using a commercial ELISA kit (HS600C, RandD systems) according to the supplier’s protocol.
CRISPRa and CRISPRi
Two activators were used, employing distinct humanized dCas9 versions (Addgene plamids 63798 and 48240). VP160 consists of 10 repeats of the p16 herpes virus activator while VPR is a fusion of p16, human herpes virus 4 (Epstein-Barr virus) replication and transcription activator Rta/BRLF and the transactivation domain of the p65 subunit of NFKB. While full-length NFKB is a known activator of IL6, VPR contains only its transactivation domain and has thus lost its ability to interact with cognate DNA sequences and effect transcription of its native targets. For CRISPRi, dCas9 fused to the Krueppel-Associated Box (KRAB) domain was transferred from Addgene plasmid 46911 into PLVX-puro (Clontech); for consistency, all constructs were introduced by transfection. Design of sgRNA for CRISPRa/i was performed via the Sequence Scan for CRISPR (SSC), using a 19 nt spacer sequence default and CRISPR activation criteria.
Western blot analyses
Whole cell extracts were lysed in SDS-free RIPA buffer (50 mM Tris-HCl, 0.15 M NaCl, 1% Triton X-100, 0.5% NaDeoxycholate, pH 7.4) supplemented with 1 X Complete Protease Inhibitor Tablets and 1 X phoSTOP cocktail (Roche). Lysates (~30 μg) were cleared by centrifugation for 2 min at 4 °C, denatured in reducing SDS-PAGE sample buffer and resolved on 1 mm thick 4–15% gradient SDS-PAGE gels; protein ladder was from Bio-Rad (Precision plus All blue). Proteins were then transferred to nitrocellulose membranes for 40 min at 100 Volts in transfer buffer containing 15% ethanol. Blocking was performed for 1 h in PBS blocking buffer (Li-Cor) diluted 1:1 with ddH20, followed by incubations with primary antibodies (1:2000) overnight. Incubations with secondary antibodies (Li-Cor; 1:20,000) were for 1 h. All antibodies were diluted in PBS and washes were performed in PBS/0.1% Tween-20. Antibodies are detailed in S1 Supplementary Materials. Acquisition and quantification was performed on an Odyssey imaging station equipped with its application software version 3.0.21.
CRISPR targeting of RP11
Deletion of exon 1 in HEK293T was accomplished by CRISPR using sgRNA sequences located within the promoter of RP11 and intron 1. Editing at the SG-505 were obtained via a similar approach, albeit using SG-505. Briefly, cells were transfected with Addgene # 71814 (eSpCas9(1.1)) alongside sgRNA expression plasmids at a mass ratio of 7/3 (Cas9/sgRNA). Single clones were then obtained by serial dilution and expanded. Editing was validated by the Guide-it Mutation detection kit (Takara Bio) and by the disappearance of a PCR product targeting the intact sgRNA site. For exon 1 targeting, 48 clones were obtained of which about half showed some evidence of editing over the targeted area; only three clones had deletions consistent with the loss of one promoter region allele. No clones harboring bi-allelic deletions of the promoter region were obtained. For SG-505 editing, 12 clones were screened, and 3 showed editing by Guide-it and 1 clone had bi-allelic editing. Editing was confirmed by sequencing of PCR amplicons obtained using oligonucleotides flanking the predicted deletion, after transfer into pCR2.1-TOPO (Thermofisher). Oligonucleotide sequences used to generate sgRNA are detailed in S1 Supplementary Materials.
RNA isolation and qRT-PCR
RNA was isolated and converted to cDNA using the High Pure Isolation Kit (Roche) and the Transcriptor First Strand cDNA Synthesis Kit (Roche), respectively. For cDNA synthesis, a 1:1 mixture of random hexamer and oligodT was used. PCR amplification and quantification was performed using the SYBR Green I Master reaction mix (Roche) on a Roche LightCycler 480. Relative amounts of target cDNAs were normalized to peptidylprolyl isomerase A (PPIA). Oligonucleotides used are described in S1 Supplementary Materials.
Statistical analysis
Unless mentioned Student’s 2-tailed t-tests were performed to test for statistical significance. Results were deemed statistically significant if p <0.05.
Online resources
Integrated DNA Technologies CRISPR-Cas9 guide RNA design: https://www.idtdna.com/site/order/designtool/index/CRISPR_PREDESIGN
CRISPRscan: https://www.crisprscan.org/
CRISPR-ERA: http://crispr-era.stanford.edu/
CCtop: https://crispr.cos.uni-heidelberg.de/
The Genetic Perturbation Platform Web Portal: https://portals.broadinstitute.org/gpp/public/analysis-tools/sgrna-design
Cas-OFFinder: http://www.rgenome.net/cas-offinder/
CROP-IT: http://www.adlilab.org/CROP-IT/cas9tool.html
UCSC genome browser: https://genome.ucsc.edu/index.html
WebGestalt: http://www.webgestalt.org/.
Supporting information
UCSC browser snapshot of conserved regions spanning RP11-326A19.4. Conservation over RP11 exons is restricted to mammals but varies with clades and species. For instance exon 1 is not found in most rodents (Euarchontoglires) but found in more distantly related Afrotheria. Exon 4 shows the highest conservation. The alternate TCONS forms (Human Body Map) reported exhibit less conservation in their last exon (red).
(PPTX)
Expression levels of genes proximal to RP11 were assessed after RP11 levels were modulated with either VP160 or KRAB in the presence of SG-286. Results are from 4 independent biological replicate (Average ± S.D.).
(PPTX)
CRISPRa targeting the MFGE8 promoter region in HEK293T cells. Numbers refer to position of sgRNA cognate site relative to start site (RefSeq: NM_001114614). Data represent the average of 3 experiments (± S.D.).
(PPTX)
Top, CRISPR editing of the SG-505 recognition region. DNA from a putative knock-out clone was PCR amplified, subcloned and individual clones sent for validation by Sanger sequencing. A total of 6 distinct sequences from that clone were obtained, conforming to 2 distinct patterns indicated above. One allele carried point mutations (red) near (-8 to -6) the PAM site while the other allele exhibited a more extensive deletion (DEL). Resulting sequences are expected to be poorly or not recognized by SG-505. Bottom, cells harboring bi-allelic editing of SG-505 binding site were transfected with CRISPRa (VPR) in the presence of either SG-505 or trcrRNA and levels of RP11 and IL6 (and PPIA) were measured 48 h post-transfection. Values are expressed relative to PPIA and then normalized to the corresponding trcrRNA values. Data represent the average values obtained from 3 passages.
(PPTX)
Specificity control for Fig 8; see Fig 8 for additional information. Levels of SRP14 were measured in CRISPRa transfected cells (in the presence of SG-505, SG-286 or trcrRNA) and normalized to the matching vehicle (0.1% DMSO) CRISPRa sample. Data represent the average of 3 experiments (± S.D.).
(PPTX)
Quantification of Western blots (Fig 9). Relative phosphorylation levels (pX/X) were measured and are normalized to the corresponding trcrRNA values (set to 1). Results represent the average of 3 biological replicates (± S.D). *, statistically different (p < 0.05) from trcrRNA.
(PPTX)
THP-1 cells, differentiated to M1 with phorbolesters (100 nM PMA, 72 h), were polarized to M1 with LPS (500 ng/ml) or vehicle (PBS, Ctl) for 2 h, and analyzed by Western blot for the presence of phosphorylated IKKA/B and IKKA. Arrowhead indicates predicted migration position of IKKA/B (84 and 87 kDa, respectively). Approximately 20 μg of THP-1 were loaded per well. HEK293T cells (30 μg, untreated) are included.
(PPTX)
Upregulation of RP11 in HuH-7 correlates with increased IL6. HuH-7 cells were transfected with the indicated sgRNA expressing constructs, along with dCAS9-VPR. Values are expressed relative to trcrRNA values, set to 1. * indicates statistically significant (p < 0.05) change with Ctl, as assessed by ANOVA. Data represent the average of 3 experiments (± S.D.).
(PPTX)
Complete searchable array results are available at the GEO repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE132451).
(XLSX)
Complete list of off-target sites predicted by CROP-it for both sgRNAs used (Tab 1:-286, Tab 2: -505).
(XLSX)
Off-target predictions were performed using Cas-OFFinder.
(XLSX)
(DOCX)
Data Availability
Transcriptome profiling on SG-286/CRISPRa transfected cells was performed at the Centre for Applied Genomics (The Hospital for Sick Children, ON, Canada). Purified RNA, was converted to cRNAs and hybridized to Human Clariom D arrays. Results were analyzed by using the Expression and Transcriptome Analysis Consoles (Applied Biosystems); Array data have been deposited at the GEO repository as GSE132451.
Funding Statement
This work was funded by a Canadian Institutes for Health Research Foundation grant (RM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Xu X, Gao J, Dai W, Wang D, Wu J, Wang J. Gene activation by a CRISPR-assisted trans enhancer. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavez A, Scheiman J, Vora S, Pruitt BW, Tuttle M, P R Iyer E, et al. Highly efficient Cas9-mediated transcriptional programming. Nat Methods. NIH Public Access; 2015;12: 326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang F, Doudna JA. CRISPR–Cas9 Structures and Mechanisms. Annu Rev Biophys. Annual Reviews; 2017;46: 505–529. [DOI] [PubMed] [Google Scholar]
- 4.Aryal NK, Wasylishen AR, Lozano G. CRISPR/Cas9 can mediate high-efficiency off-target mutations in mice in vivo. Cell Death Dis. Nature Publishing Group; 2018;9: 1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X, Scott DA, Kriz AJ, Chiu AC, Hsu PD, Dadon DB, et al. Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat Biotechnol. NIH Public Access; 2014;32: 670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Geen H, Henry IM, Bhakta MS, Meckler JF, Segal DJ. A genome-wide analysis of Cas9 binding specificity using ChIP-seq and targeted sequence capture. Nucleic Acids Res. Oxford University Press; 2015;43: 3389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horlbeck MA, Witkowsky LB, Guglielmi B, Replogle JM, Gilbert LA, Villalta JE, et al. Nucleosomes impede Cas9 access to DNA in vivo and in vitro. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deveson IW, Brunck ME, Blackburn J, Tseng E, Hon T, Clark TA, et al. Universal Alternative Splicing of Noncoding Exons. Cell Syst. 2018;6: 245–255.e5. 10.1016/j.cels.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 9.Morris K V, Mattick JS. The rise of regulatory RNA. NatRevGenet. 2014;15: 423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154: 26–46. 10.1016/j.cell.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu SJ, Horlbeck MA, Cho SW, Birk HS, Malatesta M, He D, et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science (80-). NIH Public Access; 2017;355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joung J, Engreitz JM, Konermann S, Abudayyeh OO, Verdine VK, Aguet F, et al. Genome-scale activation screen identifies a lncRNA locus regulating a gene neighbourhood. Nature. Nature Publishing Group; 2017;548: 343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paralkar VR, Taborda CC, Huang P, Yao Y, Kossenkov AV, Prasad R, et al. Unlinking an lncRNA from Its Associated cis Element. Mol Cell. Cell Press; 2016;62: 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta—Mol Cell Res. Elsevier; 2011;1813: 878–888. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. Cold Spring Harbor Laboratory Press; 2014;6: a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature. Nature Publishing Group; 2019;567: 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45: 25–33. 10.1038/ng.2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soubeyrand S, Nikpay M, Turner A, Dang A-T, Herfkens M, Lau P, et al. Regulation of MFGE8 by the intergenic coronary artery disease locus on 15q26.1. Atherosclerosis. 2019;284: 11–17. 10.1016/j.atherosclerosis.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 19.Stemmer M, Thumberger T, del Sol Keyer M, Wittbrodt J, Mateo JL. CCTop: An Intuitive, Flexible and Reliable CRISPR/Cas9 Target Prediction Tool. Maas S, editor. PLoS One. Public Library of Science; 2015;10: e0124633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno-Mateos MA, Vejnar CE, Beaudoin J-D, Fernandez JP, Mis EK, Khokha MK, et al. CRISPRscan: designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat Methods. 2015;12: 982–8. 10.1038/nmeth.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Xiao T, Chen C-H, Li W, Meyer CA, Wu Q, et al. Sequence determinants of improved CRISPR sgRNA design. Genome Res. 2015;25: 1147–1157. 10.1101/gr.191452.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Wei Z, Dominguez A, Li Y, Wang X, Qi LS. CRISPR-ERA: a comprehensive design tool for CRISPR-mediated gene editing, repression and activation. Bioinformatics. 2015;31: 3676–8. 10.1093/bioinformatics/btv423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh R, Kuscu C, Quinlan A, Qi Y, Adli M. Cas9-chromatin binding information enables more accurate CRISPR off-target prediction. Nucleic Acids Res. Narnia; 2015;43: e118–e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Cradick TJ, Brown MT, Deshmukh H, Ranjan P, Sarode N, et al. CRISPR/Cas9 systems have off-target activity with insertions or deletions between target DNA and guide RNA sequences. Nucleic Acids Res. Oxford University Press; 2014;42: 7473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The Sequence of the Human Genome. Science (80-). 2001;291: 1304–1351. [Google Scholar]
- 26.Kojima Y, Volkmer J-P, McKenna K, Civelek M, Lusis AJ, Miller CL, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536: 86–90. 10.1038/nature18935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol. 2016;64: 1403–1415. 10.1016/j.jhep.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 28.Engreitz JM, Haines JE, Perez EM, Munson G, Chen J, Kane M, et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature. 2016;539: 452–455. 10.1038/nature20149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao S, Baltimore D. The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat Immunol. NIH Public Access; 2009;10: 281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoenfelder S, Fraser P. Long-range enhancer–promoter contacts in gene expression control. Nat Rev Genet. Nature Publishing Group; 2019; 1. [DOI] [PubMed] [Google Scholar]
- 31.Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol. NIH Public Access; 2014;32: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amrani N, Gao XD, Liu P, Edraki A, Mir A, Ibraheim R, et al. NmeCas9 is an intrinsically high-fidelity genome-editing platform. Genome Biol. BioMed Central; 2018;19: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yourik P, Fuchs RT, Mabuchi M, Curcuru JL, Robb GB. Staphylococcus aureus Cas9 is a multiple-turnover enzyme. RNA. 2019;25: 35–44. 10.1261/rna.067355.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikpay M, Goel A, Won H-H, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47: 1121–30. 10.1038/ng.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]