Abstract
Background
To evaluate the efficacy of autologous platelet-rich plasma (PRP) injections in the treatment of common shoulder diseases.
Methods
The PubMed, Medline, and Central databases and trial registries were searched from their inception to October 2020 for randomized controlled trials of autologous PRP injections for shoulder diseases versus placebo or any control intervention. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines were followed in the selection, analysis, and reporting of findings. The primary outcome was pain intensity (visual analog scale), and secondary outcomes were changes in function and quality of life (QoL).
Results
A total of 17 randomized controlled trials of PRP versus control were analyzed. From 8–12 weeks to ≥1 year, PRP injections were associated with better pain relief and functional outcomes than control interventions. PRP injections were also associated with greater QoL, with an effect size of 2.61 (95% confidence interval, 2.01–14.17) at medium-term follow-up. Compared with placebo and corticosteroid injections, PRP injections provided better pain relief and functional improvement. In subgroup analyses, trials in which PRP was prepared by the double centrifugation technique, the platelet concentration in the PRP was enriched ≥5 times, leucocyte-rich PRP was used, or an activating agent was used before application reported the most effective pain relief at 6–7 months.
Conclusions
PRP injections could provide better pain relief and functional outcomes than other treatments for persons presenting with common shoulder diseases. PRP injections have a greater capacity to improve shoulder-related QoL than other interventions.
Keywords: Injections, Platelet-rich plasma, Shoulder, Pain, Meta-analysis
INTRODUCTION
Shoulder pain is one of the most common musculoskeletal symptoms in the working population, and it produces disability, decreased work efficiency, and reduced quality of life (QoL) [1]. The prevalence of shoulder pain is between 7% and 26% in the general population [2]. Most often, shoulder disease is associated with impaired function, reduced mobility, and poor mental health. Among the various causes of shoulder disease, soft tissue injuries, especially adhesive capsulitis, rotator cuff tendinopathies, rotator cuff tears, and impingement syndrome, are common. Shoulder pain and dysfunction can cause significant disability if it is not addressed effectively or the injured tissue does not heal completely.
Platelet-rich plasma (PRP) injections, which contain a mixture of heavily concentrated platelets, bioactive materials, and growth factors [3,4], have emerged as a remarkable therapy for managing sports and other musculoskeletal injuries. PRP has anti-nociceptive [5], anti-inflammatory [6], and regenerative properties [7]. Recent studies have demonstrated the use of PRP in many kinds of pathologies, from fracture-healing [8] to nerve repair [9]. Some trials have reported the superiority of PRP for pain-relief and tissue healing compared with other interventions. However, other trials have reported that PRP does not make any difference or can even make lesions worse. Despite the conflicting evidence in the literature about its efficacy, physicians are using PRP injections for increased numbers for patients who present with any kind of tissue injury, including shoulder disease. PRP therapy has some commercial interest; PRP kits are costly and manufactured by only a few medical companies. Though PRP therapy has not been included in any recommendation guidelines, many patients have already been convinced that PRP injections promote early recovery from injury and alleviate pain quickly.
Our objective in this study was to collect evidence about the magnitude of the efficacy of PRP injections in the treatment of common shoulder diseases for use in framing therapeutic guidelines. We compare the efficacy of PRP injections with that of other interventions in terms of pain relief and functional improvement in persons with shoulder diseases.
METHODS
This review was performed according to the preferred reporting items for systematic reviews and meta-analyses protocols (PRISMA-P) 2015 guidelines [10]. Institutional review board (No. T/IM-NF/PMR/20/88) permission was obtained before starting the meta-analysis and the review was registered prospectively in the international Prospective Register of Ongoing Systematic Reviews (systematic review registration – PROSPERO 2020: CRD42020199573).
Literature Search
A systematic electronic literature search was conducted in Medline, PubMed, Central, and trial registries for randomized controlled trials (RCTs) testing PRP injections against placebo injections or any control intervention in persons presenting with shoulder disease published until October 2020. Relevant keyword and MeSH terms were used during the literature search. The reference lists of eligible reports were also searched, and authors were contacted for unpublished data. The complete search strategies are available in Supplementary Material 1. Language restrictions were not applied.
Selection Criteria
All published or unpublished RCTs that compared autologous-PRP injections with placebo or another intervention for persons with shoulder diseases were eligible. Observational studies, review articles, case series, editorial comments, case reports, and animal studies were excluded.
Participants
Persons aged ≥18-years who presented with shoulder pain and dysfunction were included in this review. No restrictions were imposed on the diagnostic methods or criteria used by individual trials. Trials with fewer than eight weeks of follow-up were excluded.
Interventions
Experimental intervention
Autologous PRP injections were considered as the primary treatment for shoulder diseases in this meta-analysis. No restrictions were placed on the injection administration technique, injection frequency, injected PRP volume, PRP separation technique, or characteristics of the PRP solution. Trials in which PRP injections were used as augmentative therapies or associated with surgical/arthroscopic repair were excluded. Whole-blood injections, conditioned-serum injections, bone-marrow aspiration concentrate, stem-cells, and allogeneic-PRP were not included as experimental interventions.
Control intervention
Placebo injection or any intervention (injection/non-injection) other than PRP injection was considered as the comparator or control intervention in this review.
Outcome Measures
The outcome measurements were categorized as short term (8–12 weeks of follow-up), medium term (6–7 months of follow-up), and long term (≥1-year of follow-up).
Outcomes
The primary outcome was change in pain intensity, as assessed by a 10-cm visual analog scale (VAS). Secondary outcomes were (1) change in shoulder function, as assessed by questionnaires such as Disabilities of the Arm, Shoulder and Hand (DASH; 100 points), Shortened Disabilities of the Arm, Shoulder and Hand (QuickDASH; 100 points), the Shoulder Pain and Disability Index (SPADI; 100 points) and by the American Shoulder and Elbow Surgeons (ASES; 100 points) and Constant-Murley (100 points) scores, and (2) change in shoulder-related QoL, as assessed by the Western Ontario Rotator Cuff Index (WORC Index; 100 points).
Data Collection and Analysis
Selection of studies
The titles and abstracts of studies were screened by two reviewers (AB, KBT) who independently identified them as included, excluded, or uncertain. In case of uncertainty, the full-text article was obtained and reviewed for eligibility based on the inclusion criteria.
Data extraction and management
Two reviewers (AB, RM) independently extracted data from the included trials. Data extraction discrepancies were resolved through discussion or in consultation with a third reviewer (AM). The data extracted were the study design, etiology, participants, intervention, comparators, outcome measures, side effects, and characteristics of the PRP solution. The corresponding authors were contacted to acquire any missing data.
Data analysis
All statistical analyses were performed using RevMan 5.3 (The Cochrane Collaboration, Copenhagen, Denmark), and the meta-regression was performed using the "Metapackage” in the R programming language ver. 3.4 (The R Foundation for Statistical Computing, Vienna, Austria). All p-values were two-sided, and the significance level was fixed at p<0.05.
Assessment of the risk of bias
The methodological quality of the included trials was assessed using the Cochrane Risk of Bias tool. Two reviewers (AB, RM) independently extracted data and performed the risk-of-bias assessment; disagreements were resolved by a third reviewer (AM).
Measures of treatment effects
The outcome measures of interest, pain relief, change in shoulder function, and QoL scores, are presented as continuous data, and adverse events are presented as categorical data. Mean difference (MD) with 95% confidence intervals (CIs) were used to calculate the effect sizes of continuous outcomes measured on a standard scale (changes in pain and QoL scores), and the standardized mean difference (SMD) with corresponding 95% CIs was calculated to analyze the effect sizes of continuous outcomes measured using different standard scales (changes in shoulder function). A random-effect model was used for overall between-group analyses, irrespective of heterogeneity between individual sample sizes.
The pooled effect sizes of changes in pain (VAS) and QoL (WORC Index) were compared with their minimum clinically significant differences (MCID). MCID is defined as the slightest improvement in a treatment outcome that is perceived as necessary by the average person. The MCID for the 10-cm VAS is 1.5 cm, and that for the 100-point WORC Index is 15 points [11-13].
Assessment of heterogeneity and sensitivity analysis
Statistical heterogeneity was assessed by grouping the trials according to different control interventions/comparators. Heterogeneity across the trials was explored using the chi-square and I2 statistics. When significant heterogeneity was found, a sensitivity analysis was performed to determine how removing one or more trials affected the overall outcome result and heterogeneity.
Subgroup analysis and meta-regression
Subgroup analyses were conducted according to the site of PRP injection—sub-acromial vs. direct injection at the site of the lesion/tear vs. intra-articular—to explore the treatment associations with pain relief and functional outcomes at all follow-up durations. Subgroup analyses were also done at 6–7 months for the following subgroups: (1) pathology (rotator-cuff lesions vs. adhesive capsulitis); (2) number of injections (1 vs. >1); (3) volume of injection (≤3 mL vs. >3 mL); (4) PRP-leucocyte concentration (leucocyte-rich PRP vs. leucocyte-poor PRP); (5) PRP platelet concentration (≥5 times vs. <5 times); and (6) activating agent before PRP application (used vs. not used). Subgroup differences were considered significant if p<0.05. Meta-regressions were performed for shoulder pathology, the number of injections, the injection volume, and platelet separation techniques for the outcome of pain relief in the medium term (6–7 months).
RESULTS
Description of Included Studies
A total of 1,641 records was identified, of which 851 abstracts were screened for eligibility after removing the duplicates and irrelevant reports. Thirty-six potentially relevant full-text articles were obtained and scrutinized. Of those, 19 studies were excluded. Therefore, 17 RCTs [11,14-29] were included in this review's qualitative and quantitative analyses (Fig. 1). Among the 17 trials, five studies [11,15,17,22,23] compared PRP with placebo injections, nine studies [14,18,20,22,24-28] compared PRP with corticosteroid (CS) injections, three studies [16,18,20] compared PRP with programmed physical therapy (PT), one study [19] compared PRP with programmed exercise therapy, one study [22] compared PRP with dextrose-prolotherapy injections, one study [29] compared PRP with a hydro-dissection (HD) intervention, one study [15] compared PRP with hyaluronic acid (HA) injections, one study [15] compared PRP with a combination of HA and PRP injections, and one study [21] compared PRP with dry-needling. The PRISMA flow diagram, including reasons for excluding studies, is provided in Fig. 1.
Fig. 1.
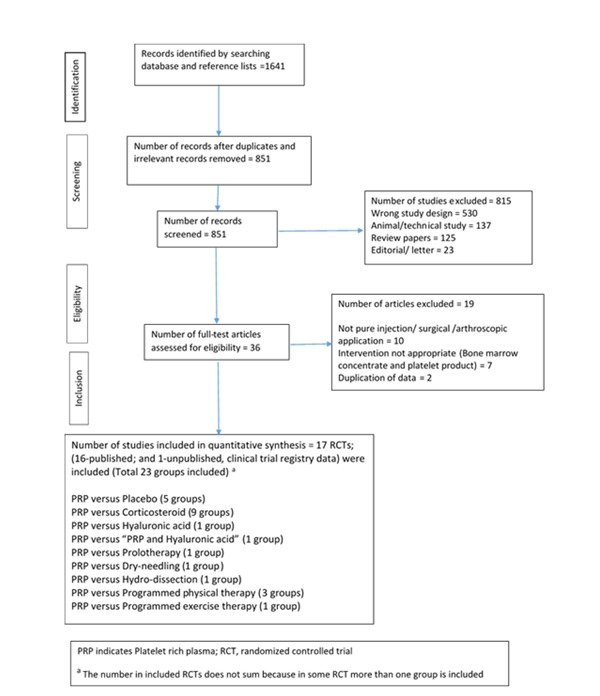
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram for the study selection process. RCT: randomized controlled trials, PRP: platelet-rich plasma. *The number in included RCTs does not sum because in some RCT more than one group is included.
Study Characteristics
The study characteristics are presented in Table 1. All studies [11,14-24,26-29] except one (NCT01123889) [25] were published between 2012 and 2020. The outcome data from the unpublished study [25] were taken from its clinical trial registry record. The sample sizes of the studies ranged from 9 [11] to 200 [15], with a total of 511 persons treated with PRP injections and 745 persons treated with placebo or control interventions. Five trials [15-17,23,29] had a total follow-up of 12 months; thirteen trials [11,14-17,19,21-24,27-29] had a follow-up of 6 months, and 17 trials [11,14-29] had a total follow-up of 8–12 weeks. The mean age of all persons was 51.34 years, and 52% of them were female. Fourteen trials [11,14-17,19-27] included persons with rotator cuff lesions, and three trials [18,28,29] included persons with adhesive capsulitis (Table 1). The cytology and other characteristics of the PRP used in the included studies are reported in Table 2.
Table 1.
Characteristics of the trials included in the review
| Study | Patient | Average age (yr) | M:F | Pathology | Average duration of symptoms | Intervention/control | Methodology | Outcome measure | Site of injection | Injection/interval | Injection technique | Injection volume (mL) | Cointervention | Adverse effect |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barreto et al. (2019) [14] | PRP, 26; | 53.3 | 36:66 | Rotator cuff impingement | NR | PRP | 6 Months, single centre, randomized, controlled, parallel group, double-blind clinical trial | UCLA, CMS, DASH (function) | Sub-acromial bursa | 1/NA | Landmark guided | 3 | No mention | No complication (only injection-related pain) |
| corticosteroid, 25 | Corticosteroid | |||||||||||||
| Cai et al. (2019) [15] | PRP, 50; | 40.6 | 99:85 | Partial-thickness tears (<1 cm) | 13.76 wk | PRP | 12 Months, multi-centres, randomized, controlled, double blind, clinical trial | Constant score, ASES (function), VAS (pain) | Sub-acromial bursa | 4/1 wk | USG-guided | 4 | No mention | Intolerance to PRP (n=2) |
| normal saline, 50; | 39.9 | NS | ||||||||||||
| SH, 50; | 38.9 | SH | ||||||||||||
| SH+PRP, 50 | 39.6 | SH+PRP | ||||||||||||
| Ilhanli et al. (2015) [16] | PRP, 30; | 59.2 | 45:17 | Partial thickness tear | 7.24 mo (mean duration) | PRP | 12 Months, single centre, randomized, controlled, parallel group, single blind, clinical trial | VAS (pain), ROM, DASH (function), Beck depression inventory | Intra-articular | 3/1 wk | NR | 6 | Home exercise program without PT | No complications |
| PT, 32 | 59.7 | PT | ||||||||||||
| Kesikburun et al. (2013) [17] | PRP, 20; | 45.5 51.4 |
13:27 | Partial thickness tear or tendinosis | >3 mo (IC) | PRP | 12 Months, single centre, Randomized, placebo controlled, double blind, clinical trial | WORC (QOL), SPADI (function), VAS (pain), passive ROM | Sub-acromial Space | 1/NA | USG-guided | 5 | Exercise program (supervised by PT), then home program | No complications (only injection-related pain) |
| placebo, 20 | Placebo (NS) injection | |||||||||||||
| Kothari (2017) [18] | PRP, 62; | 51.9 52.7 | 86:94 | Adhesive capsulitis | 4.67 mo (mean duration) | PRP | 12 Weeks, single centre, randomized, controlled, parallel group, clinical trial | ROM, VAS (pain), QuickDASH (function) | Intra-articular injection | 1/NA | Landmark guided | 2 | Home exercises | No complications |
| corticosteroid, 60; | 51.2 | CorticosteroidUST (programmed-PT) | ||||||||||||
| UST (PT), 58 | ||||||||||||||
| Nejati et al. (2017) [19] | PRP, 22; | 52.5 53.9 | 15:27 | Sub-acromial impingement syndrome | >3 mo (IC) | PRP | 6 Months, single centre, randomized controlled, parallel group, single blind, clinical trial | VAS (pain), ROM, strength, DASH (function), WORC (QOL) | Into the tear and sub-acromial bursa | 1/NA | USG-guided | 4 Total | No exercise program | No mention |
| exercise, 20 | Exercise | |||||||||||||
| Pasin et al. (2019) [20] | PRP, 30; | 49.4 47.7 | 37:53 | Sub-acromial Impingement syndrome | >3 mo (IC) | PRP | 8 Weeks, single centre, randomized controlled, parallel group, single blind, clinical Trial | VAS (pain), SDQ (function), Quick DASH (function), UCLA SRS), SF-36 (QoL) | Sub-acromial bursa injection | 1/NA | NR | 4 | Exercise program (supervised by PT) | No mention |
| corticosteroid, 30; PT, 30 | 49.9 | CorticosteroidPT | ||||||||||||
| Rha et al. (2013) [21] | PRP, 20; | 52.2 | 17:22 | Partial thickness tear (<1 cm) or tendinosis | 9.4 mo (mean duration) | PRP | 6 Months, single centre, randomized controlled, double blind, clinical trial | SPADI (function), passive ROM of shoulder, physician global rating scale | Into tear | 2/4 wk | USG-guided | 3 | Self-exercise rehabilitation program | No complications |
| dry-needling, 19 | 53.9 | Dry-needle | ||||||||||||
| Sari and Eroglu (2020) [22] | PRP, 30; | 52.1 | 43:77 | Rotator cuff lesions | 4.87 mo (mean duration) | PRP Prolotherapy Corticosteroid Lidocaine | 24 Weeks, single centre, randomized controlled, parallel group, triple blind, clinical trial | VAS (pain), ASES (function), WORC (QOL) | Sub-acromial bursa | 1/NA | USG-guided | 5 | Home exercises | No mention |
| corticosteroid, 30; | ||||||||||||||
| prolotherapy, 30; | ||||||||||||||
| lidocaine (placebo), 30 | ||||||||||||||
| Schwitzguebel et al. (2019) [23] | PRP, 41; | 48.2 | 43:35 | Interstitial supraspinatus tear | ≥6 mo (IC) | PRP | 12 Months, single centre, randomized controlled, clinical trial | Lesion-volume, VAS (pain), SANE, CMS, ASES (function) | Into tear | 2/1 mo | USG-guided | 2 | Daily activities and light sports allowed (no PT) | At 19.5 months, PRP group reported (pain >48 hr/frozen shoulder/extension of lesion) 54% compared to 26% in saline group |
| saline, 39 | 47.6 | Saline injection | ||||||||||||
| Shams et al. (2016) [24] | PRP, 20; | 52 | 21:19 | Partial-thickness tear | >3 mo (IC) | PRP | 6 Months, multi-centre, randomized controlled, clinical trial | ASES (function), CMS, Simple Shoulder Test, VAS (pain) | Sub-acromial injection | 1/NA | Landmark-guided | 2–2.5 | Home exercises without PT | No mention |
| corticosteroid, 20 | 50 | Corticosteroid | ||||||||||||
| Wongworawat (2013) [25] | PRP, 7 | 59.3 | 8:4 | Rotator cuff lesions | >4 wk (IC) | PRP | 12 Weeks, single centre, randomized controlled, clinical trial | ASES | Sub-acromial space | 1/NA | Landmark-guided | 5 | Not reported | No complications |
| corticosteroid, 5 | 59.2 | Corticosteroid | ||||||||||||
| Ibrahim et al. (2019) [26] | PRP, 15; | 46.8 | 13:17 | Rotator Cuff tendinopathy | 1.62 mo (mean duration) | PRP | 8 Weeks, single centre, randomized controlled, clinical trial | SDQ | Sub-acromial space | 1/NA | USG-guided | 2 | Home exercises | No complications |
| corticosteroid, 15 | 41.5 | Corticosteroid | ||||||||||||
| Šmíd et al. (2018) [27] | PRP, 25; | 48.7 | 31:19 | shoulder impingement syndrome | >4 wk | PRP | 6 Months, single centre, randomized controlled, parallel group, clinical trial | ASES (function), VAS (pain intensity score) | Sub-acromial space | 3/1 wk | USG-guided | 3 | Home exercises | No complications |
| corticosteroid, 25 | 50.1 | Corticosteroid | ||||||||||||
| Upadhyay et al. (2020) [28] | PRP, 60; | 47.6 46.4 | 50:70 | Adhesive capsulitis | >1 mo (IC) | PRP Corticosteroid | 6 Months, single centre, randomized controlled, single blind, parallel group, clinical trial | SPADI (function) | Intra-articular injection | 1/NA | Fluoroscope-guided | 2 | Home exercises | No complications (only injection related pain) |
| Corticosteroid, 60 | ||||||||||||||
| Wesner et al. (2016) [11] | PRP, 7; | 49.4 | 6:3 | Degenerative Tendinopathies | 62.2 mo (mean duration) | PRP | 6 Months, single centre, randomized, controlled, clinical trial | VAS (pain), DASH, WORC (QoL) | Into degenerative area of tendon/tear | 1/NA | USG-guided | 4 | Home exercises | No mention |
| Placebo, 2 | 49.5 | Saline | ||||||||||||
| Jeyaraman et al. (2018) [29] | PRP, 46; | 51.8 57.5 | 61:30 | Adhesive capsulitis | >1 mo (IC) | PRP | 12 Months, single centre, randomized controlled, parallel group, clinical trial | VAS (pain), DASH (function) | Intra-articular injection | 1/NA | Fluoroscope-guided | 3 mL PRP | Home exercises | At 1 month: with PRP, 17 reported pain and 7 swelling; with hydro-dissection, 23 reported pains |
| hydro-dissection, 45 | Hydro-dissection |
PRP: platelet-rich plasma, NR: not reported, UCLA: University of California Los Angeles, CMS: Constant-Murley score, DASH: Disabilities of the Arm, Shoulder and Hand, NA: not available, SH: sodium hyaluronate, NS: normal saline, ASES: American Shoulder and Elbow Surgeons score, VAS: visual analog scale, USG: ultrasonography, PT: physical therapy, ROM: range of motion, IC: inclusion criteria, WORC: Western Ontario Rotator Cuff Index, QoL: quality of life, SPADI: Shoulder Pain and Disability Index, UST: ultrasonic therapy, QuickDASH: Shortened DASH, SDQ: shoulder disability questionnaire, SRS: shoulder rating scale, SF-36: 36-item short form survey, SANE: Single Assessment Numeric Evaluation.
Table 2.
Characteristics of the PRP injection used in each trial
| Study | Kit | PRP preparation | Anticoagulant used | Centrifugation technique | Leucocytes concentration (LR- or LP-PRP) | Platelets concentration ×103 | Platelet concentration (compared to baseline) | Activating agent |
|---|---|---|---|---|---|---|---|---|
| Barreto et al. (2019) [14] | Coleman 80-2C Macro Centrifuge | 15 mL venous blood resulted in 3 mL PRP | NR | Double centrifugation; 1,500 RPM for 6 minutes and then 3,500 RPM for 15 minutes | LR-PRP | NR | <5 (3 times) | NR |
| Cai et al. (2019) [15] | NR | 20 mL venous blood resulted in 5–6 mL PRP | Sodium citrate | Double centrifugation; | LP-PRP | 1×1012 L–1 | NR | NR |
| 1,500 RPM for 10 minutes (4℃) followed by 2,500 RPM for 10 minutes (4℃) | ||||||||
| Ilhanli et al. (2015) [16] | NR | 15 mL venous blood resulted in 6 mL PRP | Citrate dextrose | Single centrifugation; 3,500 RPM for 9 minutes | NR | NR | <5 (2.1–2.5 times) | Calcium chloride |
| Kesikburun et al. (2013) [17] | GPS III system (Biomet Biologics) | 54 mL venous blood resulted in 6 mL PRP | Citrate | Single centrifugation; 3,200 RPM for 15 minutes | LR-PRP | (1,014.9± 340.2)×103 µL | <5 (4 times) | Nil |
| Kothari et al. (2017) [18] | Research Centrifuge REMI | 20 mL venous blood resulted in 2 mL PRP | CDPA | Single centrifugation; 200 RPM for 10 minutes | NR | NR | ≥5 (6 times) | Nil |
| Nejati et al. (2017) [19] | Tubex Autotube system (Moohan Enterprise) | 25 venous blood resulted in 5 mL PRP | Citrate dextrose | Double centrifugations; 1,300 RPM for 10 minutes followed by 2,770 RPM for 8 minutes | LR-PRP | 900,000±15,000/mm3 | <5 (3 times) | NR |
| Pasin et al. (2019) [20] | Neotec Biotechnology PRP kit (Elektro-Mag) | 8.5 mL venous blood resulted in 4 mL PRP | Citrate | Double centrifugation; 1,200 g for 5 minutes followed by 1,200 g for 10 minutes | NR | NR | NR | Nil |
| Rha et al. (2013) [21] | Prosys PRP system (Tozaiholdings) | 25 mL venous blood resulted in 3 mL PRP | Citrate dextrose | Double centrifugation; 1,600 G then 2,000g | NR | NR | NR | NR |
| Sari and Eroglu (2020) [22] | NR | 100 mL venous blood resulted in 10 mL PRP | Sodium citrate | Double centrifugation; 1,500 RPM for 6 min followed by 3,500 RPM for 12 minutes | NR | NR | ≥5 (5 times) | calcium chloride |
| Schwitzguebel et al. (2019) [23] | RegenKit BCT (Regen Lab) | 8 mL venous blood resulted in 2 mL PRP | NR | Single centrifugation; 1,500 RPM for 5 minutes | LP-PRP | (343.3±89.4) ×106/mL | <5 (1.6 times) | Nil |
| Shams et al. (2016) [24] | MyCells (ProTech, Kaylight) | 10 mL venous blood resulted in 2–2.5 mL PRP | Citrate dextrose | Single centrifugation; 3,500 RPM for 10 minutes | LR-PRP | NR | NR | NR |
| Šmíd et al. (2018) [27] | Centurion Scientific C2 | 30 mL venous blood resulted in 6 mL PRP | Citrate dextrose | Single centrifugation; | LP-PRP | 459,000/mL | <5 (2–2.5 times) | NR |
| centrifugation force of 150 g for 10 minutes at 20℃ | ||||||||
| Upadhyay et al. (2020) [28] | REMI Centrifuge C-854/6 System | 15 mL venous blood resulted in 2 mL PRP | Ethylenediaminetetraacetic acid | Single centrifugation; | NR | NR | NR | Calcium chloride |
| 1,500 RPM for 15 minutes | ||||||||
| Wesner et al. (2016) [11] | Harvest System | NR | NR | Single centrifugation; | NR | NR | NR | NR |
| centrifugation rate: NR | ||||||||
| Jeyaraman et al. (2018) [29] | Remi 8 centrifuge | NR | NR | Double centrifugation; | NR | NR | ≥5 (5–6 times) | Calcium chloride |
| 3,000 RPM for 10 minutes and 5,000 RPM for 10 minutes | ||||||||
| Ibrahim et al. (2019) [26] | NR | 20 mL venous blood resulted in 2 mL PRP | Sodium citrate | Double centrifugation; | NR | NR | NR | Thrombin |
| 700–1,500 RPM for 15–20 minutes and 2,500-3,500 RPM for 10 minutes | ||||||||
| Wongworawat (2013) [25] | Magellan Autologous Platelet Separator System | 45 mL venous blood resulted in 5 mL PRP | NR | NR | NR | NR | NR | NR |
PRP: platelet-rich plasma, LR: leucocyte rich, LP: leucocytes poor, NR: not reported, RPM: revolutions per minute, CDPA: Citrate Phosphate Dextrose Adenine Solution.
A variety of functional outcome measures were used in the included trials: SPADI (3 RCTs) [17,21,28], DASH (5 RCTs) [11,14,16,19,29], QuickDASH (2 RCTs) [18,20], ASES score (6 RCTs) [15,22,24,25,27,29], a shoulder disability questionnaire [26], and a single assessment numeric evaluation [23]. The risk of bias in each trial has been summarized in Table 3. Among the 17 RCTs, 12 trials (71%) [14-19,21-24,27,28] adequately generated randomized sequences, seven (41%) [14,17,19,21,23,24,28] adequately concealed allocation, five (29%) [14,17,21-23] adequately blinded participants, and 10 (59%) [15-23,28] blinded outcome assessors.
Table 3.
Risk of bias summary for the included studies
| Study | Random sequence generation | Allocation concealment | Blinding of participants | Blinding of personnel | Blinding of outcome assessors | Incomplete outcome data | Selective reporting | Other potential sources of bias |
|---|---|---|---|---|---|---|---|---|
| Barreto et al. (2019) [14] | Low risk | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Cai et al. (2019) [15] | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Low risk |
| Ibrahim et al. (2019) [26] | High risk | Unclear risk | High risk | High risk | Unclear risk | Low risk | Low risk | Low risk |
| Ilhanli et al. (2015) [16] | Low risk | Unclear risk | High risk | High risk | Low risk | Low risk | Low risk | Low risk |
| Jeyaraman et al. (2018) [29] | High risk | High risk | High risk | High risk | Unclear risk | Low risk | Low risk | Low risk |
| Kesikburun et al. (2013) [17] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Kothari et al. (2017) [18] | Low risk | High risk | High risk | High risk | Low risk | Low risk | Low risk | Low risk |
| Nejati et al. (2017) [19] | Low risk | Low risk | High risk | High risk | Low risk | Low risk | Low risk | Low risk |
| Pasin et al. (2019) [20] | Unclear risk | Unclear risk | High risk | High risk | Low risk | Low risk | Low risk | Low risk |
| Rha et al. (2013) [21] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Low risk | Low risk |
| Sari and Eroglu (2020) [22] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Schwitzguebel et al. (2019) [23] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Shams et al. (2016) [24] | Low risk | Low risk | High risk | High risk | High risk | Unclear risk | Low risk | Low risk |
| Šmíd et al. (2018) [27] | Low risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| Upadhyay et al. (2020) [28] | Low risk | Low risk | High risk | High risk | Low risk | Low risk | Low risk | Low risk |
| Wesner et al. (2016) [11] | Unclear risk | High risk | Unclear risk | Unclear risk | Unclear risk | High risk | High risk | Low risk |
| Wongworawat (2013) [25] | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | High risk | High risk | Unclear risk |
Effects of Intervention
Pain relief
Short-term follow-up (8–12 weeks)
Evidence from eight RCTs (14 groups, 978 participants) [15,17-20,22,26,27] suggests that although increased short-term pain relief was reported with the PRP injections, the difference with the control interventions was not significant (MD, 0.26 cm; 95% CI, –0.19 to 0.71; I2=89%; p=0.25) (Fig. 2). Similarly, the subgroup analysis found that PRP injections were not significantly better than placebo injections (MD, 0.28 cm; 95% CI, –0.05 to 0.61; I2=0%, p=0.1) or CS injections (MD, 0.41 cm; 95% CI, –0.20 to 1.01; I2=83%, p=0.19) in reducing shoulder pain (Fig. 2). Supplementary Fig. 1 illustrates the short-term pain relief according to the different sites of PRP injection.
Fig. 2.
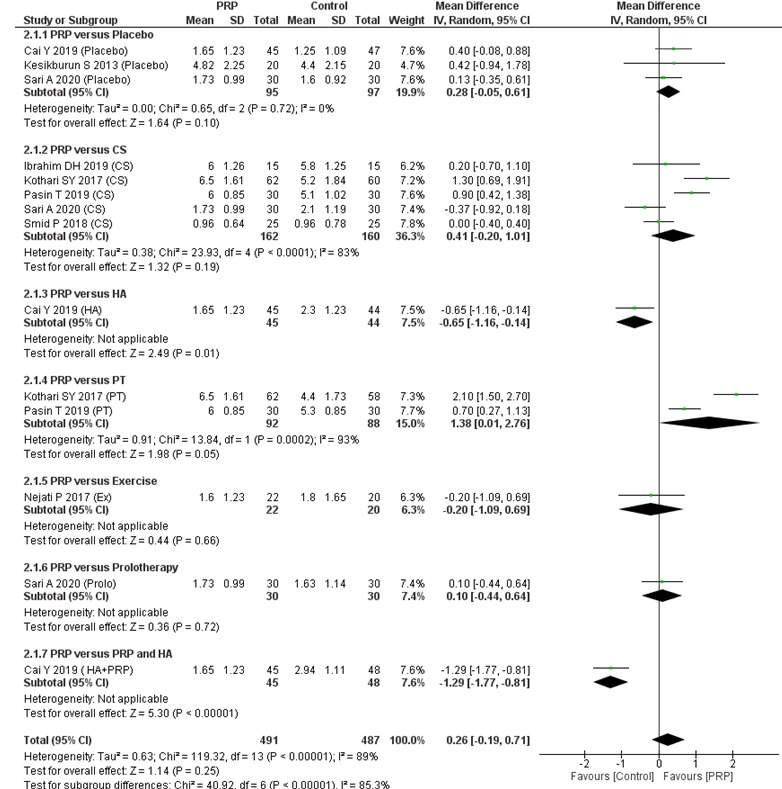
Forest plot of the included studies pooled using a random-effects model to assess short-term (8–12 weeks) pain relief: comparison between PRP injections and control interventions. The forest plot was acquired from meta-analyses of detailed data about differences in visual analog scale pain scores. The vertical line indicates no difference between the intervention groups. PRP: platelet-rich plasma, SD: standard deviation, IV: inverse variance, CI: confidence interval, CS: corticosteroid, HA: hyaluronic acid, PT: physical therapy, Ex: programmed exercise therapy, HA+PRP: combination of hyaluronic acid and platelet-rich plasma.
Medium-term follow-up (6–7 months)
The medium-term follow-up results from eight RCTs (12 groups) [6,9-12,15,22,23] showed greater pain relief with PRP injection compared with the control interventions (MD, 1.00 cm; 95% CI, 0.35–1.65; I2=93%, p=0.002) (Fig. 3). Though the difference in pain relief was statistically significant, the weighted MD of medium-term pain-relief did not reach the MCID target of 1.5 cm on the 10-cm VAS. Similarly, compared to placebo (MD, 1.64 cm; 95% CI, 0.40–2.87; I2=90%) and CS injections (MD, 0.81 cm; 95% CI, 0.10–1.51; I2= 75%), PRP injections were associated with greater pain relief with marginal significance (Fig. 3). Supplementary Fig. 2 illustrates the medium-term pain relief according to the different sites of PRP injection.
Fig. 3.
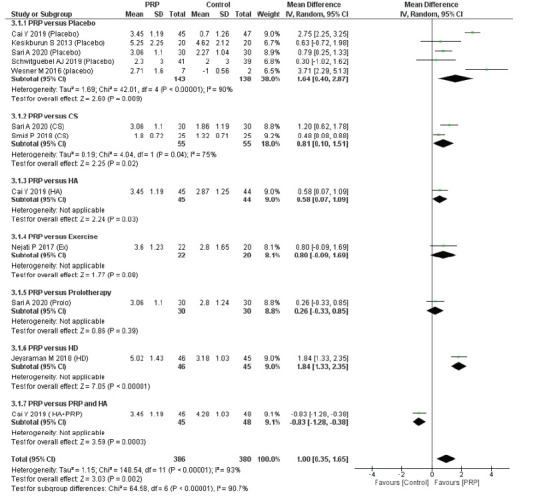
Forest plot of the included studies pooled using a random-effects model to assess medium-term (6–7 months) pain relief: comparison between PRP injections and control interventions. The forest plot was acquired from meta-analyses of detailed data about differences in visual analog scale pain scores. The vertical line indicates no difference between the intervention groups. PRP: platelet-rich plasma, SD: standard deviation, IV: inverse variance, CI: confidence interval, CS: corticosteroid, HA: hyaluronic acid, Ex: programmed exercise therapy, HD: hydro-dissection, HA+PRP: combination of hyaluronic acid and platelet-rich plasma.
Long-term follow-up (≥1 year)
Similar to the short-term follow-up results, the long-term follow-up results indicate that PRP injection provided increased pain relief, but the difference with the control interventions was not significant (MD, 1.12 cm; 95% CI, –0.58 to 2.82; I2=98%) (Fig. 4). Supplementary Fig. 3 illustrates long-term pain relief according to the different sites of PRP injection.
Fig. 4.
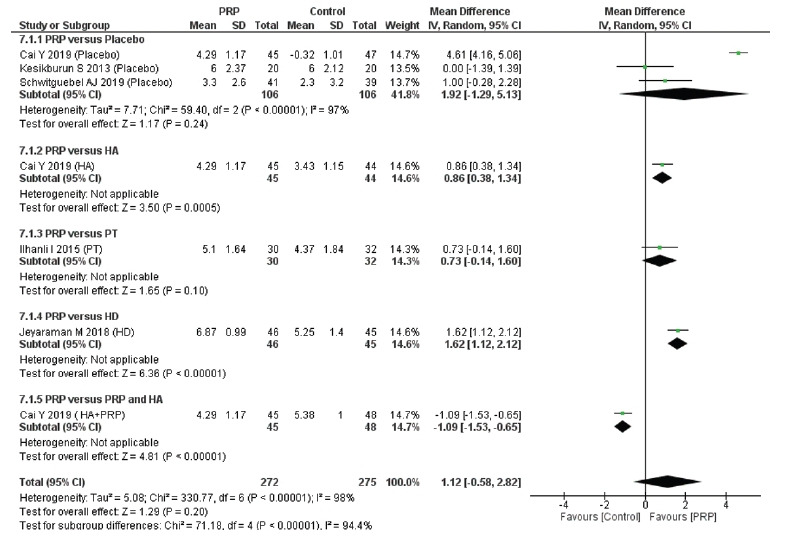
Forest plot of the included studies pooled using a random-effects model to assess long-term (≥1 year) pain relief: comparison between PRP injections and control interventions. The forest plot was acquired from meta-analyses of detailed data about differences in visual analog scale pain scores. The vertical line indicates no difference between the intervention groups. PRP: platelet-rich plasma, SD: standard deviation, IV: inverse variance, CI: confidence interval, HA: comparator hyaluronic acid, PT: physical therapy, HD: comparator hydro-dissection, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma.
Functional outcomes
Overall, PRP injection was associated with slightly better functional outcomes than the control interventions, but the differences were not significant in the short term (SMD, 0.24 points; 95% CI, –0.30 to 0.78) (Supplementary Fig. 4), medium term (SMD, 0.50 points; 95% CI, –0.13 to 1.14) (Supplementary Fig. 5), or long term (SMD, 1.22 points; 95% CI, –0.44 to 2.89) (Supplementary Fig. 6). Supplementary Figs. 7-9 illustrate the short-, medium-, and long-term functional outcomes, respectively, according to the different sites of PRP injection.
When PRP injections were compared with only placebo injections, they were found to be superior in the short term (SMD, 0.79 points; 95% CI, –0.95 to 2.53) (Supplementary Fig. 4), medium term (SMD, 1.36 points; 95% CI, –0.21 to 2.92) (Supplementary Fig. 5), and long term (SMD, 2.52 points; 95% CI, –0.72 to 5.76) (Supplementary Fig. 6). In functional outcomes, PRP injection was found to be better than CS injection in the short term (SMD, 0.44 points; 95% CI, –0.10 to 0.97; 9 RCTs) (Supplementary Fig. 4) and medium term (SMD, 0.41 points; 95% CI, –0.12 to 0.94; 5 RCTs) (Supplementary Fig. 5). However, none of those differences (SMD) in functional outcome scores between PRP and CS or placebo injections was statistically significant (<0.05).
Changes in QoL
Evidence from four RCTs (six groups) [11,17,19,22] suggests that compared with control interventions, PRP injections were associated with statistically greater improvements in shoulder disease–specific QoL (WORC Index) in the short term (MD, 3.47-points; 95% CI, –0.21 to 7.14; I2=56%) (Supplementary Fig. 10) and medium term (MD, 8.09-points; 95% CI, 2.01 to 14.17; I2=55%) (Supplementary Fig. 11). No pooled analysis was done for long-term follow up because only one study reported long-term WORC Index scores. However, the MCID for WORC Index is 15 points, so those statistical differences aren’t clinically meaningful.
Safety outcomes
Adverse events were assessed in 12 trials [14-18,21,23,25-29]. Of them, nine trials [14,16-18,21,25-28] found no serious adverse events after PRP injection. Only three trials [15,23,29] reported adverse events other than injection-associated pain. Among the reported events, one trial [15] reported intolerance to the injection, and another trial [23] reported the development of frozen shoulder and tear pejoration after injection.
Additional analyses
The subgroup analyses (Table 4) found that trials using the double centrifugation technique [14,15,19-22,26,29], a platelet activating agent [16,22,26,28,29], or image-guided injections [11,15,17,19,21-23,26-29] were associated with greater medium-term (6–7 months) pain relief (p<0.05) than the respective control interventions. A meta-regression analysis suggested that the effect-size in medium-term (6–7 months) pain relief did not differ significantly with the underlying shoulder pathology (slope coefficient Standard Error [SE], 2.586; p=0.27), number of injections (single vs. ≥2; Standard Error [SE], 1.09; p=0.81), volume of injection (≤3 mL vs. >3 mL; Standard Error [SE], 1.83; p=0.28), site of injection (sub-acromial vs. intra-articular vs. others; Standard Error [SE], 0.55; p=0.48], or centrifugation technique (single vs. double; Standard Error [SE], 1.32; p=0.36).
Table 4.
Subgroup analysis of personal and study-related factors affecting medium-term (6–7 months) pain relief with PRP vs. control interventions
| Comparison | No. of groups | MD (95% CI) | p-value |
|---|---|---|---|
| Shoulder pathology | <0.05 | ||
| Rotator cuff lesions | 9 | 0.90 (0.31 to 1.49) | |
| Adhesive capsulitis | 1 | 1.84 (1.33 to 2.35) | |
| No. of injections | <0.05 | ||
| 1 | 5 | 0.75 (0.41 to 1.10) | |
| >1 | 5 | 1.23 (0.27 to 2.20) | |
| PRP-leucocyte concentration | <0.05 | ||
| LR-PRP | 2 | 0.75 (0.01 to 1.49) | |
| LP-PRP | 4 | 1.06 (–0.15 to 2.28) | |
| PRP platelet concentration | <0.05 | ||
| ≥5× | 4 | 1.03 (0.37 to 1.69) | |
| <5× | 4 | 0.52 (0.19 to 0.86) | |
| Platelet separation technique | <0.05 | ||
| Single centrifugation | 3 | 0.48 (0.11 to 0.84) | |
| Double centrifugation | 7 | 1.19 (0.50 to 1.87) | |
| Platelet activating agent | <0.05 | ||
| Used | 4 | 1.03 (0.37 to 1.69) | |
| Not used | 3 | 0.48 (0.11 to 0.84) | |
| Injection volume | <0.05 | ||
| ≤3 mL/injection | 3 | 0.94 (–0.13 to 2.01) | |
| >3mL/injection | 7 | 1.03 (0.30 to 1.75) |
Analysis was done after exclusion of study by Wongworawat (unpublished trial), Wesner et al.’s study (total sample was very less; total 7) [11], and one group of Cai et al.’s study (where PRP was compared with combination of PRP and hyaluronic acid) [15].
PRP: platelet rich plasma, MD: mean difference, LR: leucocyte rich, LP: leucocytes poor.
DISCUSSION
This review revealed that PRP injection resulted in larger improvements in clinical outcome measures (VAS pain scores, functional outcomes, and QoL scores) in common shoulder diseases, but those improvements differed at the short, medium, and long term. Compared with the control interventions, PRP injections provided significant pain reduction and improvements in QoL scores only at the medium term (6–7 months). In this meta-analysis, interventions such as dry-needling injection and HD were categorized as different control interventions instead as placebo. Injection dry-needling and HD are used as active interventions for managing shoulder pain, and their mechanisms of action are entirely different from placebo injections. In the subgroup analyses, PRP injections were found to offer superior pain relief and functional outcomes compared with placebo and CS. However, those differences were only significant in the medium term (6–7 months).
Studies in which (1) repeat PRP injections were given (≥2-injections) at intervals for a single pathology; (2) the PRP solution was prepared by the double centrifugation technique; (3) leucocyte-rich PRP was used instead of leucocyte-poor PRP; (4) a platelet activating agent was used after PRP preparation; or (5) the platelet concentrations in the PRP solution were at least five times greater than in whole blood all reported significant pain relief in the medium term (6–7 months).
Injection techniques can be categorized by injection site. Assuming that the mechanism of action would be different at different sites, persons with shoulder pain were sorted into four categories: (1) injection into the sub-acromial bursa [14,15,17,20,22,24-27], (2) injection at the site of the lesion/tear [11,21,23], (3) injection into the sub-acromial bursa and the site of the lesion [19], and (4) injection into the joint (intra-articular) [16,18,28,29].
Therapeutic exercises are essential for the rehabilitation of sports and other musculoskeletal injuries. In this review, all of the trials [11,16-18,20-22,24,26-29] (except two [19,23]) encouraged their participants to do some degree of home exercise, along with the PRP injection. Therefore, a certain degree of exercise along with PRP injection is essential to obtain the optimal effect in shoulder disease.
Irrespective of etiology, pain and impaired shoulder function are the main clinical features of shoulder diseases. Depending on the stage of tendinopathy, tears or capsular fibrosis, rotator cuff lesions, and adhesive capsulitis can be at different stages. Tears, especially rotator cuff tears, can be partial or complete. None of the trials included in this meta-analysis administered a PRP injection as a primary intervention into the site of a complete tear.
Persons with shoulder disease usually present with similar clinical features: shoulder pain, restricted range of motion, and reduced shoulder-related QoL [14-30]. The injection techniques used to treat shoulder diseases are usually limited to three sites (intra-articular, inside the bursa, or at the site of the lesion). Therefore, in this meta-analysis, we included all persons presenting with shoulder pain and impaired function, irrespective of pathology. This approach allowed us to review many RCTs and more effectively judge the pain relief and functional outcomes of PRP injections in shoulder diseases.
In the literature, several reviews [31-34] have examined the role of PRP therapy as an adjunct treatment used during or after surgical repair of ligament injuries. However, reviews of PRP injections as a primary or standalone intervention in shoulder diseases have been minimal. To date, only three reviews [35-37] have examined the efficacy of PRP injection on rotator cuff tendinopathy. However, those reviews did not include common shoulder diseases such as frozen shoulder or calcific rotator cuff lesions. Frozen shoulder is a widespread cause of shoulder pain and disability, and PRP injections are increasingly being used to treat it. Furthermore, those reviews [35-37] considered only five trials with short follow-up durations.
A few previous reviews [3,37-40] have considered PRP injections for tendon and ligament injuries to the upper and lower limbs, which partially overlaps with this analysis. Miller et al. [38] and Chen et al. [3] conducted systematic reviews of PRP injections used to treat all types of soft tissue injuries. Miller et al. [38], Lin et al. [37], and Chen et al. [3] reported significant pain reduction and better functional outcomes after PRP treatment. However, we also found reviews [35,39,40] that failed to demonstrate any benefit of PRP treatment over other treatments in pain-relief scores for tendon and ligament injuries in all time frames.
Our data suggests that in common shoulder diseases PRP injections are better than other interventions for pain relief and functional recovery. The strengths of this review are as follows. (1) We performed an extensive literature search for eligible trials. All published and unpublished trials were included in this review. (2) Irrespective of shoulder pathology, all persons presenting with shoulder pain and dysfunction were included. (3) Sensitivity analyses were performed to address methodological differences among studies. (4) All relevant clinical outcome scales were included to acquire comprehensive effects (pain, shoulder function, and shoulder-related QoL) after PRP injection. (5) Short- and long-term efficacy was assessed.
It is important to note that this review also has some limitations. First, there was significant heterogeneity among the RCTs. Different shoulder pathologies, PRP preparation techniques, injection techniques, injection sites, injection administrations, and a mixed variety of controls were used in the included trials. There was also a lack of information on the cytology of the prepared PRP. None of the studies evaluated growth factors after preparing PRP. The studies underreported rotator cuff lesion/tear sizes, the grade of rotator cuff tendinopathy, and the stages of adhesive capsulitis. Second, although 17 RCTs were included, the number of RCTs in each subgroup meta-analysis was small for most control interventions. Most of the subgroups findings were based on only a single RCT. Therefore, the treatment-effect estimates for pain relief and functional outcomes should be explained very carefully because further research has a great chance of significantly changing them. Third, the RCTs included in the analysis suffered from methodological limitations. Many of them lacked proper concealments and blinding. Especially for RCTs in which programmed PT and exercise programs were provided, it was almost impossible to blind the participants for autologous-PRP injections. All those factors need to be considered when interpreting our results. An RCT comparing cell counts (platelets, leucocyte counts, and growth factor assessment) and different kits would be of great interest in the future.
This review suggests that PRP injections might provide better pain relief and functional outcomes than other treatments for persons presenting with shoulder diseases. At 6–7 months, PRP injections have a greater capacity to reduce shoulder pain and improve shoulder disease-specific QoL than other treatments. However, these findings are not strong enough to allow us to recommend for or against the use of PRP injections. More homogeneous, high-quality evidence from large, robust RCTs is required.
Footnotes
Financial support
None.
Conflict of interest
None.
Supplementary Material
Supplementary materials can be found via https://doi.org/10.5397/cise.2021.00353.
Identification of trials and search method
Forest plot of the included studies grouped according to the sites of injection and pooled using a random-effects model to assess short-term (8–12 weeks) pain relief: comparison between platelet-rich plasma (PRP) injections and control interventions. The vertical line indicates no difference between the intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, CS: comparator corticosteroid, PT: comparator programmed physical therapy, Ex: comparator programmed exercise therapy.
Forest plot of the included studies grouped according to the sites of injection and pooled using a random-effects model to assess medium-term (6–7 months) pain relief: comparison between platelet-rich plasma (PRP) injections and control interventions. The vertical line indicates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, CS: comparator corticosteroid, Ex: comparator programmed exercise therapy, HD: hydro-dissection.
The forest plot for included studies, sub-grouped according to sites of injection, pooled together using a random-effects model for assessing long-term (≥1 year) pain-relief: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, CS: comparator corticosteroid, PT: comparator programmed physical therapy, CS: comparator corticosteroid, HD: hydro-dissection.
The forest plot for included studies pooled together using a random-effects model for assessing short-term (8–12weeks) functional outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; CS: comparator corticosteroid, HA: comparator hyaluronic acid, PT: comparator programmed physical therapy, Ex: comparator programmed exercise therapy.
The forest plot for included studies pooled together using a random-effects model for assessing medium-term (6–7 months) functional outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; CS: comparator corticosteroid, HA: comparator hyaluronic acid, Ex: comparator programmed exercise therapy, HD: hydro-dissection.
The forest plot for included studies pooled together using a random-effects model for assessing at ≥1-year functional outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; HA: comparator hyaluronic acid, PT: comparator programmed physical therapy, HD: hydro-dissection.
The forest plot for included studies, sub-grouped according to sites of injection, pooled together using a random-effects model for assessing short-term (8–12 weeks) functional outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; CS: comparator corticosteroid, HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, PT: comparator programmed physical therapy, Ex: comparator programmed exercise therapy.
The forest plot for included studies, sub-grouped according to sites of injection, pooled together using a random-effects model for assessing medium-term (6–7 months) functional outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; CS: comparator corticosteroid, HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, Ex: comparator programmed exercise therapy.
The forest plot for included studies, sub-grouped according to sites of injection, pooled together using a random-effects model for assessing long-term (≥1 year) functional outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, PT: comparator programmed physical therapy, HD: hydro-dissection.
The forest plot for included studies pooled together using a random-effects model for assessing long-term (8–12 weeks) QoL (WORC-index) outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, CS: comparator corticosteroid, Ex: comparator programmed exercise therapy.
The forest plot for included studies pooled together using a random-effects model for assessing long-term (6–7 months) QoL (WORC-Index) outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; CS: comparator corticosteroid, Ex: comparator programmed exercise therapy, Prolo: comparator prolotherapy.
REFERENCES
- 1.Roquelaure Y, Ha C, Leclerc A, Touranchet A, Sauteron M, Melchior M, Imbernon E, Goldberg M. Epidemiologic surveillance of upper-extremity musculoskeletal disorders in the working population. Arthritis Rheum. 2006;55:765–78. doi: 10.1002/art.22222. [DOI] [PubMed] [Google Scholar]
- 2.van den Dolder PA, Ferreira PH, Refshauge KM. Effectiveness of soft tissue massage and exercise for the treatment of non-specific shoulder pain: a systematic review with meta-analysis. Br J Sports Med. 2014;48:1216–26. doi: 10.1136/bjsports-2011-090553. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Jones IA, Park C, Vangsness CT., Jr The Efficacy of Platelet-Rich Plasma on Tendon and Ligament Healing: A Systematic Review and Meta-analysis With Bias Assessment. Am J Sports Med. 2018;46:2020–32. doi: 10.1177/0363546517743746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao HT, Marra KG, Rubin JP. Application of platelet-rich plasma and platelet-rich fibrin in fat grafting: basic science and literature review. Tissue Eng Part B Rev. 2014;20:267–76. doi: 10.1089/ten.teb.2013.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee HR, Park KM, Joung YK, Park KD, Do SH. Platelet-rich plasma loaded hydrogel scaffold enhances chondrogenic differentiation and maturation with up-regulation of CB1 and CB2. J Control Release. 2012;159:332–7. doi: 10.1016/j.jconrel.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Bendinelli P, Matteucci E, Dogliotti G, Corsi MM, Banfi G, Maroni P, Desiderio MA. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J Cell Physiol. 2010;225:757–66. doi: 10.1002/jcp.22274. [DOI] [PubMed] [Google Scholar]
- 7.Strauss FJ, Nasirzade J, Kargarpoor Z, Stähli A, Gruber R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: a systematic review of in vitro studies. Clin Oral Investig. 2020;24:569–84. doi: 10.1007/s00784-019-03156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simman R, Hoffmann A, Bohinc RJ, Peterson WC, Russ AJ. Role of platelet-rich plasma in acceleration of bone fracture healing. Ann Plast Surg. 2008;61:337–44. doi: 10.1097/SAP.0b013e318157a185. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Jin Z, Wang J, Chen S, Hu Y, Ren L, Wang Y, Song Q, Tian X, Xie F, Peng J, Peng N, Luo Y, Wang Y. Ultrasound-guided platelet-rich plasma injection and multimodality ultrasound examination of peripheral nerve crush injury. NPJ Regen Med. 2020;5:21. doi: 10.1038/s41536-020-00101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesner M, Defreitas T, Bredy H, Pothier L, Qin Z, McKillop AB, Gross DP. A Pilot Study Evaluating the Effectiveness of Platelet-Rich Plasma Therapy for Treating Degenerative Tendinopathies: A Randomized Control Trial with Synchronous Observational Cohort. PLoS One. 2016;11:e0147842. doi: 10.1371/journal.pone.0147842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18:205–7. doi: 10.1136/emj.18.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Witte PB, Henseler JF, Nagels J, Vliet Vlieland TP, Nelissen RG. The Western Ontario rotator cuff index in rotator cuff disease patients: a comprehensive reliability and responsiveness validation study. Am J Sports Med. 2012;40:1611–9. doi: 10.1177/0363546512446591. [DOI] [PubMed] [Google Scholar]
- 14.Barreto RB, Azevedo AR, Gois MC, Freire MRM, Silva DS, Cardoso JC. Platelet-Rich Plasma and Corticosteroid in the Treatment of Rotator Cuff Impingement Syndrome: Randomized Clinical Trial. Rev Bras Ortop (Sao Paulo) 2019;54:636–43. doi: 10.1016/j.rboe.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai YU, Sun Z, Liao B, Song Z, Xiao T, Zhu P. Sodium Hyaluronate and Platelet-Rich Plasma for Partial-Thickness Rotator Cuff Tears. Med Sci Sports Exerc. 2019;51:227–33. doi: 10.1249/MSS.0000000000001781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilhanli I, Guder N, Gul M. Platelet-Rich Plasma Treatment With Physical Therapy in Chronic Partial Supraspinatus Tears. Iran Red Crescent Med J. 2015;17:e23732. doi: 10.5812/ircmj.23732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kesikburun S, Tan AK, Yilmaz B, Yaşar E, Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013;41:2609–16. doi: 10.1177/0363546513496542. [DOI] [PubMed] [Google Scholar]
- 18.Kothari SY, Srikumar V, Singh N. Comparative Efficacy of Platelet Rich Plasma Injection, Corticosteroid Injection and Ultrasonic Therapy in the Treatment of Periarthritis Shoulder. J Clin Diagn Res. 2017;11:RC15–8. doi: 10.7860/JCDR/2017/17060.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nejati P, Ghahremaninia A, Naderi F, Gharibzadeh S, Mazaherinezhad A. Treatment of Subacromial Impingement Syndrome: Platelet-Rich Plasma or Exercise Therapy? A Randomized Controlled Trial. Orthop J Sports Med. 2017;5:2325967117702366. doi: 10.1177/2325967117702366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasin T, Ataoğlu S, Pasin Ö, Ankarali H. Comparison of the Effectiveness of Platelet-Rich Plasma, Corticosteroid, and Physical Therapy in Subacromial Impingement Syndrome. Arch Rheumatol. 2019;34:308–16. doi: 10.5606/ArchRheumatol.2019.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rha DW, Park GY, Kim YK, Kim MT, Lee SC. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil. 2013;27:113–22. doi: 10.1177/0269215512448388. [DOI] [PubMed] [Google Scholar]
- 22.Sari A, Eroglu A. Comparison of ultrasound-guided platelet-rich plasma, prolotherapy, and corticosteroid injections in rotator cuff lesions. J Back Musculoskelet Rehabil. 2020;33:387–96. doi: 10.3233/BMR-191519. [DOI] [PubMed] [Google Scholar]
- 23.Schwitzguebel AJ, Kolo FC, Tirefort J, Kourhani A, Nowak A, Gremeaux V, Saffarini M, Lädermann A. Efficacy of Platelet-Rich Plasma for the Treatment of Interstitial Supraspinatus Tears: A Double-Blinded, Randomized Controlled Trial. Am J Sports Med. 2019;47:1885–92. doi: 10.1177/0363546519851097. [DOI] [PubMed] [Google Scholar]
- 24.Shams A, El-Sayed M, Gamal O, Ewes W. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837–42. doi: 10.1007/s00590-016-1826-3. [DOI] [PubMed] [Google Scholar]
- 25.Wongworawat MD. ClinicalTrials.gov; 2014. Treatment of rotator cuff syndrome with injection of autologous platelet rich plasma [Internet] [cited 2021 Aug 7]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT01123889. [Google Scholar]
- 26.Ibrahim DH, El-Gazzar NM, El-Saadany HM, El-Khouly RM. Ultrasound-guided injection of platelet rich plasma versus corticosteroid for treatment of rotator cuff tendinopathy: effect on shoulder pain, disability, range of motion and ultrasonographic findings. Egypt Rheumatologist. 2019;41:157–61. [Google Scholar]
- 27.Šmíd P, Hart R, Komzák M, Paša L, Puskeiler M. Treatment of the shoulder impingement syndrome with PRP injection. Acta Chir Orthop Traumatol Cech. 2018;85:261–5. [PubMed] [Google Scholar]
- 28.Upadhyay S, Jorule K, Varma HS, Chansoria M. Ongoing efficacy of platelet-rich plasma vs corticosteroid injection in patients with adhesive capsulitis: a prospective randomized assessor-blind comparative analysis. J Recent Adv Pain. 2020;6:10–6. [Google Scholar]
- 29.Jeyaraman M, Ramesh R, Prajwal GS, Dhamsania HJ. The comparative and prospective study on Efficacy and functional outcome of autologous platelet rich plasma injection vs hydrodissection in adhesive capsulitis of shoulder. Int J Res Orthop. 2018;4:848–53. [Google Scholar]
- 30.Barman A, Mukherjee S, Sahoo J, Maiti R, Rao PB, Sinha MK, Sahoo D, Tripathy SK, Patro BK, Bag ND. Single Intra-articular Platelet-Rich Plasma Versus Corticosteroid Injections in the Treatment of Adhesive Capsulitis of the Shoulder: A Cohort Study. Am J Phys Med Rehabil. 2019;98:549–57. doi: 10.1097/PHM.0000000000001144. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Xu M, Guo W, Wang Y, Zhao S, Zhong L. Clinical efficacy and safety of platelet-rich plasma in arthroscopic full-thickness rotator cuff repair: A meta-analysis. PLoS One. 2019;14:e0220392. doi: 10.1371/journal.pone.0220392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han C, Na Y, Zhu Y, Kong L, Eerdun T, Yang X, Ren Y. Is platelet-rich plasma an ideal biomaterial for arthroscopic rotator cuff repair? A systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2019;14:183. doi: 10.1186/s13018-019-1207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samy TM, Khater AH, Ahmed AK. Systematic review of literature on the efficiency of using PRP in arthroscopic rotator cuff repair. QJM. 2020;113(Suppl 1):hcaa059.013. [Google Scholar]
- 34.Hurley ET, Lim Fat D, Moran CJ, Mullett H. The Efficacy of Platelet-Rich Plasma and Platelet-Rich Fibrin in Arthroscopic Rotator Cuff Repair: A Meta-analysis of Randomized Controlled Trials. Am J Sports Med. 2019;47:753–61. doi: 10.1177/0363546517751397. [DOI] [PubMed] [Google Scholar]
- 35.Hurley ET, Hannon CP, Pauzenberger L, Fat DL, Moran CJ, Mullett H. Nonoperative Treatment of Rotator Cuff Disease With Platelet-Rich Plasma: A Systematic Review of Randomized Controlled Trials. Arthroscopy. 2019;35:1584–91. doi: 10.1016/j.arthro.2018.10.115. [DOI] [PubMed] [Google Scholar]
- 36.Lin MT, Chiang CF, Wu CH, Huang YT, Tu YK, Wang TG. Comparative Effectiveness of Injection Therapies in Rotator Cuff Tendinopathy: A Systematic Review, Pairwise and Network Meta-analysis of Randomized Controlled Trials. Arch Phys Med Rehabil. 2019;100:336–49. doi: 10.1016/j.apmr.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 37.Lin MT, Wei KC, Wu CH. Effectiveness of Platelet-Rich Plasma Injection in Rotator Cuff Tendinopathy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diagnostics (Basel) 2020;10:189. doi: 10.3390/diagnostics10040189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller LE, Parrish WR, Roides B, Bhattacharyya S. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017-000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheth U, Simunovic N, Klein G, Fu F, Einhorn TA, Schemitsch E, Ayeni OR, Bhandari M. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94:298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 40.Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;2014:CD010071. doi: 10.1002/14651858.CD010071.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identification of trials and search method
Forest plot of the included studies grouped according to the sites of injection and pooled using a random-effects model to assess short-term (8–12 weeks) pain relief: comparison between platelet-rich plasma (PRP) injections and control interventions. The vertical line indicates no difference between the intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, CS: comparator corticosteroid, PT: comparator programmed physical therapy, Ex: comparator programmed exercise therapy.
Forest plot of the included studies grouped according to the sites of injection and pooled using a random-effects model to assess medium-term (6–7 months) pain relief: comparison between platelet-rich plasma (PRP) injections and control interventions. The vertical line indicates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, CS: comparator corticosteroid, Ex: comparator programmed exercise therapy, HD: hydro-dissection.
The forest plot for included studies, sub-grouped according to sites of injection, pooled together using a random-effects model for assessing long-term (≥1 year) pain-relief: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, CS: comparator corticosteroid, PT: comparator programmed physical therapy, CS: comparator corticosteroid, HD: hydro-dissection.
The forest plot for included studies pooled together using a random-effects model for assessing short-term (8–12weeks) functional outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; CS: comparator corticosteroid, HA: comparator hyaluronic acid, PT: comparator programmed physical therapy, Ex: comparator programmed exercise therapy.
The forest plot for included studies pooled together using a random-effects model for assessing medium-term (6–7 months) functional outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; CS: comparator corticosteroid, HA: comparator hyaluronic acid, Ex: comparator programmed exercise therapy, HD: hydro-dissection.
The forest plot for included studies pooled together using a random-effects model for assessing at ≥1-year functional outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; HA: comparator hyaluronic acid, PT: comparator programmed physical therapy, HD: hydro-dissection.
The forest plot for included studies, sub-grouped according to sites of injection, pooled together using a random-effects model for assessing short-term (8–12 weeks) functional outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; CS: comparator corticosteroid, HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, PT: comparator programmed physical therapy, Ex: comparator programmed exercise therapy.
The forest plot for included studies, sub-grouped according to sites of injection, pooled together using a random-effects model for assessing medium-term (6–7 months) functional outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; CS: comparator corticosteroid, HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, Ex: comparator programmed exercise therapy.
The forest plot for included studies, sub-grouped according to sites of injection, pooled together using a random-effects model for assessing long-term (≥1 year) functional outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, PT: comparator programmed physical therapy, HD: hydro-dissection.
The forest plot for included studies pooled together using a random-effects model for assessing long-term (8–12 weeks) QoL (WORC-index) outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; HA: comparator hyaluronic acid, HA+PRP: comparator combination of hyaluronic acid and platelet-rich plasma, CS: comparator corticosteroid, Ex: comparator programmed exercise therapy.
The forest plot for included studies pooled together using a random-effects model for assessing long-term (6–7 months) QoL (WORC-Index) outcome: comparison between platelet-rich plasma (PRP) injection and control interventions. The vertical line demonstrates no difference between the two intervention groups. SD: standard deviation, IV: inverse variance, CI: confidence interval; CS: comparator corticosteroid, Ex: comparator programmed exercise therapy, Prolo: comparator prolotherapy.


