Abstract
Objective
This study was undertaken to characterize antiseizure medication (ASM) treatment pathways in Medicare beneficiaries with newly treated epilepsy.
Methods
This was a retrospective cohort study using Medicare claims. Medicare is the United States' federal health insurance program for people aged 65 years and older plus younger people with disabilities or end‐stage renal disease. We included beneficiaries with newly treated epilepsy (International Classification of Diseases codes for epilepsy/convulsions 2014–2017, no ASM in the previous 2 years). We displayed the sequence of ASM fills using sunburst plots overall, then stratified by mood disorder, age, and neurologist prescriber. We tabulated drug costs for each pathway.
Results
We included 21 458 beneficiaries. Levetiracetam comprised the greatest number of pill days (56%), followed by gabapentin (11%) and valproate (8%). There were 22 288 unique treatment pathways. The most common pathways were levetiracetam monotherapy (43%), gabapentin monotherapy (10%), and valproate monotherapy (5%). Gabapentin was the most common second‐ and third‐line ASM. Whereas only 2% of pathways involved first‐line lacosamide, those pathways accounted for 19% of cost. Gabapentin and valproate use was increased and levetiracetam use was decreased in beneficiaries with mood disorders compared to beneficiaries without mood disorders. Levetiracetam use was increased and gabapentin, valproate, lamotrigine, and topiramate use was decreased in beneficiaries aged >65 years compared with those aged 65 years or less. Lamotrigine, levetiracetam, and lacosamide use was increased and gabapentin use was decreased in beneficiaries whose initial prescriber was a neurologist compared to those whose prescriber was not a neurologist.
Significance
Levetiracetam monotherapy was the most common pathway, although substantial heterogeneity existed. Lacosamide accounted for a small percentage of ASMs but a disproportionately large share of cost. Neurologists were more likely to prescribe lamotrigine compared with nonneurologists, and lamotrigine was prescribed far less frequently than may be endorsed by guidelines. Future work may explore patient‐ and physician‐driven factors underlying ASM choices.
Keywords: antiseizure medications, epilepsy, medication pathways
Key Points.
We evaluated treatment pathways in newly treated beneficiaries with epilepsy in Medicare
Levetiracetam was the most common first‐line and gabapentin the most common second/third‐line medication
Lamotrigine, levetiracetam, and lacosamide use was increased in beneficiaries whose initial prescriber was a neurologist
Lacosamide was disproportionately expensive compared to its small share of pill days
Lamotrigine was prescribed less frequently than may be currently endorsed by guidelines in older adults
1. INTRODUCTION
The number of available antiseizure medications (ASMs) has rapidly expanded over the past few decades. 1 Although head‐to‐head trials have compared efficacy and tolerability between some ASMs, 2 there is no single clearly best ASM. 3 , 4 Consequently, clinicians must tailor ASM selection to the individual patient. For example, older adults are at heightened risk for adverse drug effects related to multimorbidity, bone health, polypharmacy, cardiovascular risk, and altered elimination pharmacokinetics. 5 , 6 Given these unique considerations, and that first‐generation enzyme‐inducing ASMs such as phenytoin and carbamazepine are known to exert drug–drug interactions and adverse effects such as cognitive dysfunction, 7 recent studies have favored lamotrigine or levetiracetam over carbamazepine in terms of tolerability or efficacy for older adults. 8 , 9
Scarce work exists about the actual sequence of ASM usage in real‐world settings. Large‐scale data 10 found levetiracetam to be the most common ASM filled by Medicare beneficiaries. However, that study only examined the most common ASMs in 2008–2010 in ~3700 selected minority‐enriched populations and did not examine the longitudinal sequence of ASMs used within any particular patient. Thus, gaps remain in terms of understanding not only the most common first‐line ASM choices, but also the most common ASMs chosen after first‐line treatment failure in a more updated broader population. Only about 50% of patients with epilepsy will have their seizures controlled by their first ASM, 11 and thus describing the most common ASMs in terms of only a single cross‐section may not fully describe treatment choices made over time for any given patient.
In this study, we examined individual patient‐level ASM treatment pathways (the first line, second line, third line) among a larger, more updated sample of newly treated Medicare beneficiaries with epilepsy. We described the most common sequences of ASMs overall, plus stratified by several clinically relevant patient‐ and physician‐related dimensions. Additionally, given that prior work has demonstrated wide cost variation across ASMs and found that pharmaceuticals are the costliest component of neurologic care, 12 we evaluated the drug costs of each treatment pathway.
2. MATERIALS AND METHODS
2.1. Study design and dataset
This was a retrospective cohort study of people with epilepsy identified in a 20% random sample of fee‐for‐service Medicare administrative claims data. In total, data including baseline, eligibility, and follow‐up periods spanned 2012–2019. Medicare is the United States' federal health insurance program for people aged 65 years and older in addition to younger people with disabilities or end‐stage renal disease. Medicare covers inpatient (Part A) and outpatient (Part B) care as well as prescription drugs (Part D). 13 We obtained physician information from the 2013 American Medical Association Physician Professional Data Masterfile, which contains demographics and training information regarding 1 001 536 US physicians.
2.2. Procedures involving human subjects
This study was deemed exempt by the University of Michigan Institutional Review Board. No additional patient consent was necessary.
2.3. Patient selection
We identified our cohort similarly to prior work. 14 To identify epilepsy cases, we required at least one International Classification of Disease (ICD) epilepsy code (ICD‐9 before October 1, 2015: 345.xx; ICD‐10 after October 1, 2015: G40) at any point or else at least two convulsion codes separated by at least 30 days (ICD‐9 before October 1, 2015: 780x.; ICD‐10 after October 1, 2015: R56), plus at least one ASM fill (ASMs are listed in Table S1), during 2014–2017. We included all individuals qualifying for Medicare (at least 65 years old, with disability, and/or with end‐stage renal disease). To identify newly treated epilepsy cases, we required that the first ASM during the study period was prescribed when or after the first ICD code was assigned, and that beneficiaries had at least 2 years of continuous Part D eligibility prior to their first ASM fill, with no ASM fill during that 2‐year window. To follow ASMs over time, we included beneficiaries who also were continuously enrolled in Part D for 2 years after their first ASM fill or until death. To calculate comorbidities and acute care utilization, we also restricted analyses to beneficiaries who were continuously enrolled in Parts A/B (and not C, given their claims would not appear in our dataset) during the year they filled their first ASM.
Combining ICD codes (considering both emergent vs. nonemergent site of care) with ASM fills in Medicare as we have done identifies patients with epilepsy with an area under the curve of .93, sensitivity of 88%, and specificity of 98%. 15 More generally, ICD codes have been found to have a positive predictive value of 85%–99%, 16 and adding ASMs to ICD codes as we have done (as opposed to using ICD codes alone) increases the positive predictive value further for identifying epilepsy cases. 17 Nonetheless, to further improve positive predictive value, 18 we performed a sensitivity analysis where we further required at least two epilepsy codes (not considering convulsion codes) during 2014–2017 in addition to all other inclusion criteria to increase confidence that we were including patients being treated after epileptic seizures as opposed to epilepsy mimics or prophylactic treatment.
2.4. Variables
We defined a treatment pathway 19 as the sequence of ASMs filled during the study period. A treatment pathway could be comprised of a single ASM if the beneficiary filled only one unique ASM over the observation period. If a patient filled more than one ASM, their treatment pathway was the sequence of ASMs filled in the order that they were filled (e.g., levetiracetam, then lamotrigine). If a beneficiary filled more than one new ASM on a given date (e.g., levetiracetam, then lamotrigine/topiramate on the same date), in our main analysis they were assigned more than one pathway (e.g., levetiracetam then lamotrigine, and also levetiracetam then topiramate), one for each combination. However, we performed a sensitivity analysis restricted to those beneficiaries with only a single newly filled ASM on any given date.
We described baseline variables for each beneficiary using data from the year of their first ASM fill. Variables included age, sex, race (White, Black, Hispanic, Asian), Medicaid dual eligibility, rural ZIP code, 20 reason for Medicare entitlement (age, disability, end‐stage renal disease), region of the United States according to the US Census (South, Midwest, Northeast, West), 21 epilepsy type (i.e., focal vs. generalized 22 ), possible epilepsy etiologies (e.g., stroke, intracranial hemorrhage, traumatic brain injury) and a limited number of comorbidities (particularly mood dysfunction, because we anticipated levetiracetam to be the most common ASM) and Charlson Comorbidity Index 23 , 24 (a weighted sum of 22 comorbidities, where higher scores indicate greater comorbidity), both identified by ICD codes (Table S2), number of unique medications including but not limited to ASMs, total prescription drug costs (total price paid for the drug at the point of sale including ingredient cost, dispensing fee, and sales tax, all adjusted to 2018 dollars using the gross domestic product implicit price deflator adjusting all years to 2018 dollars 25 to adjust health expenditures in terms of purchasing power from the societal perspective 26 ), and the number of emergency room plus inpatient visits (acute care visits).
We a priori hypothesized that prescribing could differ between neurologists and nonneurologists, and therefore identified the single physician who prescribed the greatest number of the beneficiary's ASM prescriptions and pill supply in the year of their first ASM fill, based on their National Provider Index and merged in whether the Masterfile indicated their specialty was neurology.
2.5. Statistical analysis
We described baseline variables using medians and interquartile ranges (IQRs), or frequencies (%).
We tabulated the most common treatment pathways in terms of count and frequency. We primarily displayed this information using a sunburst plot. A sunburst plot consists of concentric rings, where the inner ring represents the first chosen ASM. If a beneficiary filled only a single ASM, they would contribute data only to the inner ring. If a beneficiary then also subsequently filled a second ASM, they would also contribute data to the next innermost ring, and so forth. We limited to three rings and only those ASMs filled by at least 10 patients for display. The relative size of each component is proportional to the count represented by each ASM. We then repeated the main sunburst plot, but weighted the data by cost per day throughout the entire pathway (how expensive a pathway is), instead of our main analyses above that represented the number of beneficiaries per pathway (how common a pathway is).
We prespecified several stratified sunburst plots. Given previous work has shown that levetiracetam is the most common ASM prescribed to Medicare beneficiaries 10 , 14 , 27 and mood dysfunction is the primary relevant potential adverse effect, 28 we stratified according to the presence or absence of mood disorders (Table S2 lists considered ICD codes) in the year of the first ASM fill. As a sensitivity analysis, given levetiracetam could cause mood disorders or preexisting mood disorders could influence whether levetiracetam was prescribed, we stratified according to presence of a mood disorder in the year before the first ASM fill (i.e., if the first ASM prescription appeared in 2014, then we stratified by whether a mood disorder was noted in 2013). Next, given the special importance of older age in terms of the potential for adverse events and age > 65 years being an important eligibility criterion for Medicare, we stratified by age ≤ 65 versus > 65 years. Because specialists could have different practice patterns than nonspecialists, we stratified according to whether the initial prescriber was a neurologist.
Because certain ASM combinations may have particularly synergistic effects in terms of effectiveness (e.g., lamotrigine plus valproate 29 ) or tolerability (e.g., similar mechanism of action), we reported on several particular overlapping combinations. We counted ASM #2 as overlapping with ASM #1 if the first appearance of ASM #2 occurred after the first appearance of ASM #1, and before pill supplies ran out for ASM #1 (determined by the last fill date of ASM #1 plus its number of days of supply). We considered the following sodium channel blockers as representative of ASMs with a similar mechanism of action: carbamazepine, eslicarbazepine, lamotrigine, lacosamide, oxcarbazepine, phenytoin, and rufinamide. 30
2.6. Data availability statement
All datasets are available to purchase at https://www.resdac.org/. Aggregated deidentified data may be shared upon request.
3. RESULTS
There were 21 458 beneficiaries who met criteria for newly treated epilepsy (Figure 1). The median age was 72 years (IQR = 61–81), 55% were female, and 79% were White (Table 1).
FIGURE 1.
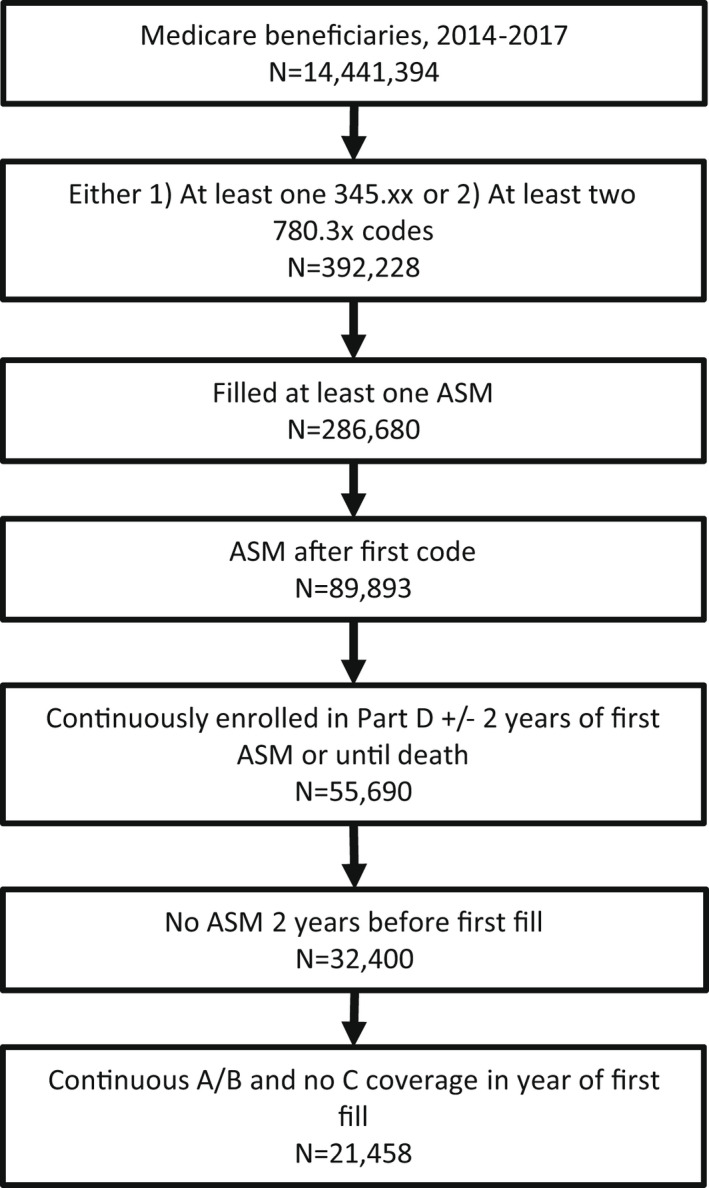
Patient flowchart. ASM, antiseizure medication
TABLE 1.
Population description (N = 21 458)
| Characteristic | Median or n (IQR or %) |
|---|---|
| Year of first antiseizure medication fill | |
| 2014 | 4958 (23%) |
| 2015 | 5568 (26%) |
| 2016 | 5594 (26%) |
| 2017 | 5338 (25%) |
| Age, years | 72 (61–81) |
| Female sex | 11 740 (55%) |
| Race | |
| White | 16 434 (79%) |
| Black | 3487 (17%) |
| Hispanic | 617 (3%) |
| Asian | 343 (2%) |
| Dual eligible for Medicaid | 12 874 (60%) |
| Rural ZIP code | 5923 (28%) |
| Reason for entitlement | |
| Age | 15 108 (70%) |
| Disability | 6248 (29%) |
| End‐stage renal disease | 253 (1%) |
| Region | |
| South | 8531 (42%) |
| Midwest | 4867 (24%) |
| Northeast | 3720 (18%) |
| West | 3431 (17%) |
| Epilepsy type | |
| Focal | 4595 (21%) |
| Generalized | 3582 (17%) |
| Both | 1381 (6%) |
| Unclassified | 11 900 (55%) |
| Comorbidities | |
| Cardiac arrest | 517 (2%) |
| Dementia | 7251 (34%) |
| Intracranial hemorrhage | 2997 (14%) |
| Ischemic stroke | 8332 (39%) |
| Meningoencephalitis | 524 (2%) |
| Mood disorder | 7219 (34%) |
| Mood disorder in prior year | 4128 (19%) |
| Traumatic brain injury | 1742 (8%) |
| Tumor, CNS | 852 (4%) |
| Charlson Comorbidity Index | |
| 0 | 4096 (19%) |
| 1–3 | 10 008 (47%) |
| 4–6 | 5270 (25%) |
| 7+ | 2084 (10%) |
| Unique medications, n | 13 (9–18) |
| Acute care visits | |
| 0 | 2253 (10%) |
| 1 | 4792 (22%) |
| 2+ | 14 413 (67%) |
| Any neurologist visit | 16 525 (77%) |
| Neurologist as primary ASM prescriber | 5513 (31%) |
Patient variables refer to the year when each beneficiary filled their first antiseizure medication (2014–2017) unless otherwise stated.
Abbreviations: ASM, antiseizure medication; CNS, central nervous system; IQR, interquartile range.
Table 2 displays the most common ASMs overall and according to their sequence. Levetiracetam comprised the greatest number of pill days (56%), followed by gabapentin (11%) and valproate (8%). Levetiracetam was the most common first‐line choice, whereas gabapentin was the most common second‐ and third‐line choice. Despite lacosamide only comprising 3% of all pill days, it comprised the greatest pill cost (39%).
TABLE 2.
Number and percent of pill days by medication
| ASM | % of all pill days | % of all cost | #1, n (%) | #2, n (%) | #3, n (%) |
|---|---|---|---|---|---|
| Denominator | 10 626 500 pill days | $20 339 416 for all pills | 22 288 pathways | 6560 pathways with a second‐line ASM | 1665 pathways with a third‐line ASM |
| Levetiracetam | 56% | 20% | 13 111 (59%) | 1114 (17%) | 144 (9%) |
| Gabapentin | 11% | 2% | 2900 (13%) | 1553 (24%) | 295 (18%) |
| Valproate | 8% | 9% | 1641 (7%) | 869 (13%) | 237 (14%) |
| Phenytoin | 6% | 4% | 1549 (7%) | 458 (7%) | 93 (6%) |
| Lamotrigine | 6% | 4% | 824 (4%) | 697 (11%) | 223 (13%) |
| Lacosamide | 3% | 39% | 512 (2%) | 480 (7%) | 145 (9%) |
| Topiramate | 2% | 1% | 492 (2%) | 327 (5%) | 113 (7%) |
| Oxcarbazepine | 2% | 1% | 352 (2%) | 257 (4%) | 106 (6%) |
| Carbamazepine | 1% | 2% | 314 (1%) | 154 (2%) | 58 (3%) |
| Pregabalin | 1% | 7% | 217 (1%) | 326 (5%) | 103 (6%) |
| Phenobarbital | 1% | <1% | 124 (1%) | 33 (1%) | 14 (1%) |
| Zonisamide | 1% | <1% | 107 (<1%) | 124 (2%) | 60 (4%) |
| Primidone | 1% | <1% | 104 (<1%) | 99 (2%) | 28 (2%) |
| Eslicarbazepine | <1% | 4% | 19 (<1%) | 35 (1%) | 26 (2%) |
| Perampanel | <1% | <1% | 5 (<1%) | 1 (<1%) | 4 (<1%) |
| Clobazam | <1% | 2% | 4 (<1%) | 12 (<1%) | 6 (<1%) |
| Ethosuximide | <1% | <1% | 4 (<1%) | 1 (<1%) | — |
| Brivaracetam | <1% | 1% | 3 (<1%) | 15 (<1%) | 4 (<1%) |
| Everolimus | <1% | 2% | 2 (<1%) | 2 (<1%) | 2 (<1%) |
| Tiagabine | <1% | <1% | 2 (<1%) | 1 (<1%) | 1 (<1%) |
| Felbamate | <1% | <1% | 1 (<1%) | 1 (<1%) | 1 (<1%) |
| Rufinamide | <1% | <1% | 1 (<1%) | 1 (<1%) | 1 (<1%) |
| Ezogabine | <1% | <1% | — | — | 1 (<1%) |
Abbreviation: ASM, antiseizure medication.
There were 22 288 ASM pathways among these 21 458 beneficiaries (given that a beneficiary could fill more than one ASM on a given date), including 1015 unique pathways. The most common unique ASM pathways (Table 3) were levetiracetam monotherapy (43%), gabapentin monotherapy (10%), and valproate monotherapy (5%). No other treatment pathway comprised >5% of the population.
TABLE 3.
Most common treatment pathways
| First | Second | Third | n | % of 22 288 |
|---|---|---|---|---|
| Levetiracetam | 9663 | 43% | ||
| Gabapentin | 2231 | 10% | ||
| Valproate | 1125 | 5% | ||
| Phenytoin | 867 | 4% | ||
| Levetiracetam | Gabapentin | 857 | 4% | |
| Lamotrigine | 510 | 2% | ||
| Levetiracetam | Valproate | 462 | 2% | |
| Levetiracetam | Lamotrigine | 381 | 2% | |
| Topiramate | 284 | 1% | ||
| Lacosamide | 272 | 1% | ||
| Phenytoin | Levetiracetam | 269 | 1% | |
| Levetiracetam | Lacosamide | 240 | 1% | |
| Levetiracetam | Phenytoin | 235 | 1% | |
| Oxcarbazepine | 210 | 1% | ||
| Carbamazepine | 178 | 1% | ||
| Gabapentin | Levetiracetam | 169 | 1% | |
| Gabapentin | Pregabalin | 157 | 1% | |
| Valproate | Levetiracetam | 144 | 1% | |
| Pregabalin | 140 | 1% |
Only those comprising at least 1% of all pathways are shown here. Each row represents a pathway, and this table displays how many beneficiaries had a sequence of antiseizure medication fill following each pathway.
Figure 2 and Figures [Link], [Link], [Link], [Link], [Link] display sunburst plots. Substantial variation existed in treatment pathways. In the main analysis (Figure 2, left), 663 of 1015 (65%) pathways had only a single beneficiary with that sequence of ASMs. We repeated our main sunburst plot for those 20 672 (96%) beneficiaries with only one new ASM filled on any given date (Figure S1, left), and for those 15 310 (71%) beneficiaries with at least two 345.xx/G40.xx codes (Figure S1, right). Results were largely similar. We weighted the plot by cost per pill day (Figure 2, right). Whereas only 2% of pathways involved first‐line lacosamide, those pathways accounted for 19% of costs.
FIGURE 2.
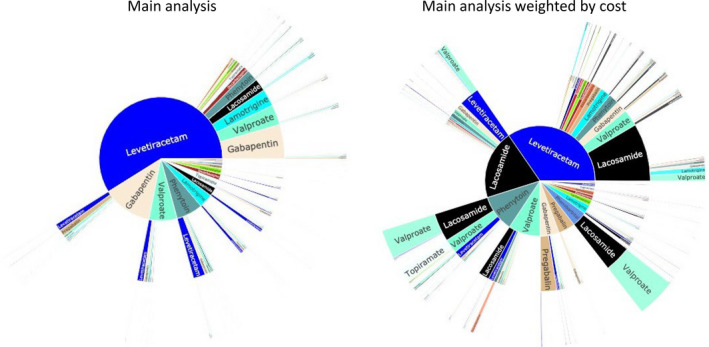
Unstratified sunburst plots. Left: Main population and main analysis (n = 1281 unique pathways). Right: Main population, but weighted by cost per pill day, whereas all other plots in this article were weighted by the sample size
We then stratified pathways according to several prespecified patient‐ or physician‐level variables (Figures [Link], [Link], [Link], [Link]). Levetiracetam was the most common first‐line choice in all subgroups. Gabapentin and valproate use was increased and levetiracetam use was decreased in beneficiaries with mood disorders compared to beneficiaries without mood disorders (Figure S2). Results were similar when stratified by presence of a mood disorder diagnosis in the year before the first ASM fill (Figure S3). Levetiracetam use was increased and gabapentin, valproate, lamotrigine, and topiramate use was decreased in beneficiaries aged >65 years compared with those aged 65 years or less (Figure S4). Lamotrigine, levetiracetam, and lacosamide use was increased and gabapentin use was decreased in beneficiaries whose initial prescriber was a neurologist compared to those whose initial prescriber was not a neurologist (Figure S5).
We tabulated the frequency of several ASM combinations. There were 17 150 (80%) beneficiaries who filled neither lamotrigine nor valproate, 4079 (19%) who filled either lamotrigine or valproate but not both, 150 (1%) who filled both lamotrigine and valproate at any point during the study, and 79 (53% of 150; <1% of all beneficiaries) who filled valproate and lamotrigine overlapping each other.
There were 15 923 (74%) who did not fill any sodium channel blocker, 4776 (22%) who filled one unique sodium channel blocker, 655 (3%) who filled two, 97 (.5%) who filled three, and seven (.03%) who filled four at any point during the study. Of the 655 + 97 + 7 = 759 who filled at least two unique sodium channel blockers, 301 (40% of 759; 1% of all beneficiaries) filled at least two overlapping each other.
4. DISCUSSION
We performed a retrospective study of ASM treatment pathways (the sequence of ASM choices) in newly treated epilepsy among Medicare beneficiaries during 2014–2017 and followed all beneficiaries for 2 years after the first filled ASM. Levetiracetam was the most common first‐line ASM, and the most common pathway involved levetiracetam monotherapy. Substantial heterogeneity existed in terms of ASM choice and mechanism of action, although gabapentin was the most common second‐ and third‐line choice, and we found other differences according to patient factors (e.g., mood dysfunction) and physician factors (neurologist vs. nonneurologist). Although lacosamide accounted for only 3% of pill days and only 2% of pathways used lacosamide as first‐line therapy, lacosamide accounted for 39% of pill cost and its first‐line pathways accounted for 19% of costs.
Many ASM choices are available for patients with newly treated epilepsy. Previous practice guidelines released in 2004 recommended several options such as gabapentin, lamotrigine, topiramate, and oxcarbazepine for monotherapy in newly diagnosed epilepsy. 31 Those guidelines were updated in 2018 (toward the end of our observation period, which spanned maximally until 2019), 4 recommending lamotrigine for patients at least 60 years old, and lamotrigine (Level B: probably effective) and levetiracetam (Level C: possibly effective) for new onset focal epilepsy in adults, and finding a lack of high‐quality studies pertaining to a large number of other US Food and Drug Administration‐approved ASMs. There were no Level A (established as effective) recommendations, and thus lamotrigine was the most strongly endorsed first‐line ASM. Nonetheless, in our sample of predominantly older adults, lamotrigine constituted only 6% of pill days, was the first‐line choice in only 4% of beneficiaries (3% in those aged >65 years), was the second‐ or third‐line choice in only 11%–13% of cases, and was even less common among older compared with younger adults. Despite evidence suggesting synergistic efficacy of lamotrigine plus valproate, 29 only 79 beneficiaries had overlapping fills of both ASMs despite ~6800 filling more than one ASM at any point. Although it is known that valproate increases lamotrigine levels, requiring caution during titration regarding risk of Stevens–Johnson syndrome, it is also possible that such rare synergistic use may be a lost therapeutic opportunity if titrated appropriately. Valproate and phenytoin both were more common first‐line choices than lamotrigine, despite their known cognitive adverse effects and drug–drug interactions. 7 One could theorize that lamotrigine was a less common first‐line choice because of its slow dose escalation schedule, but future work could explore reasons behind prescribing preferences. Levetiracetam was the most common ASM and does have many advantages, including ability for more rapid titration; limited drug interactions, which is particularly relevant in Medicare's aging population; intravenous to oral equivalence, overall favorable tolerability profile, and generic availability. We observed that levetiracetam was less commonly used in beneficiaries with mood disorders compared to beneficiaries without mood disorders, as may be expected, yet it still ranked far more common than lamotrigine even among beneficiaries with mood disorders. Our stratified plots showed that neurologists tend to prescribe a greater share of lamotrigine and a smaller share of phenytoin and valproate as first‐line ASMs compared with nonneurologists, which one could theorize may reflect greater familiarity with related guidelines and evidence, although it also possible that differences in comorbidity mixtures could also explain different prescribing patterns. Further work is needed to explore why the current guideline's only Level B or higher recommendation has been pursued so infrequently.
One striking finding was the disproportionate share of cost due to lacosamide (39% of ASM costs) compared with its relatively infrequent prevalence (3% of pill days). Lacosamide does share many of the same advantages as other newer generation ASMS such as levetiracetam. However, the most recent guidelines suggest no high‐quality studies to support adjunct lacosamide over less costly drugs in terms of efficacy 4 ; thus, potential benefits must be weighed against its increased cost. Lacosamide was brand‐only throughout this study period, although its patent is set to expire in the United States in 2022, after which point cost per pill may decrease with the introduction of a generic substitution.
A key strength of our study was its large national population‐based cohort capturing real‐world longitudinal ASM usage, particularly capturing older patients with newly treated epilepsy. However, our study has numerous limitations. First, our data may not generalize to younger epilepsy patients or the privately insured older population without Part D coverage, for whom issues such as cost and comorbidities could differ substantially. Nonetheless, Medicare covers approximately 18% of the US population. 32 Second, identifying epilepsy cases in administrative datasets using ICD codes risks misclassification. 33 For example, many ASMs have nonepilepsy indications such as pain or mood and thus to the extent our data includes beneficiaries without true epilepsy, our pathways could have overestimated the frequency of such ASMs (e.g., gabapentin) in populations with epilepsy, and it is possible that beneficiaries could have filled ASMs at some point prior to the start of our study window. Medicare data also do not contain electroencephalographic results to strictly apply the International League Against Epilepsy's definition of epilepsy based on recurrence risk. 34 Nonetheless, recent work has suggested good sensitivity (up to 88%) and specificity (98%) of Medicare data compared with chart review epilepsy diagnoses. 15 Although limited Medicare‐specific data have suggested poorer positive predictive value of codes, more generally work has suggested high positive predictive values when combining codes plus ASMs as we have done, 16 and we conducted a further sensitivity analysis requiring at least two epilepsy codes without including any convulsion‐only codes. 17 , 18 Third, it is not possible to capture all of the large number of clinical dimensions that may influence treatment decisions. We prespecified several potentially important dimensions (i.e., mood dysfunction given we anticipated that levetiracetam would be the most common ASM, age given its importance in terms of side effects and Medicare eligibility, and neurologist prescriber). Future work may continue to explore drivers of treatment decisions, and the primary purpose of our study was to describe pathways at large with several representative prespecified subgroup analyses chosen for their theoretical importance. Fourth, p‐value‐based hypothesis tests are difficult to apply to sunburst plots; thus, judgments about the importance of differences between groups are qualitative.
5. CONCLUSIONS
We found that levetiracetam monotherapy was the most common ASM, with gabapentin as the most common second‐ and third‐line choice. Lacosamide accounted for a relatively small percentage of pill days but a disproportionately large share of cost, and lamotrigine appeared to be prescribed less frequently than existing practice guidelines might seem to endorse, particularly among nonneurologists. Future work may further explore patient‐ and physician‐driven factors underlying ASM choices to better align practice with evidence‐based guidelines.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTIONS
All coauthors were substantially involved in the study and the preparation of the manuscript. No undisclosed groups or persons have had a primary role in the study and/or in manuscript preparation. All coauthors have seen and approved the submitted version of the paper and accept responsibility for its content.
Supporting information
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Table S1‐S2
ACKNOWLEDGMENTS
S.W.T. is supported by the Susan S. Spencer Clinical Research Training Scholarship and the Michigan Institute for Clinical and Health Research J Award (UL1TR002240). R.E.Y. is supported by the Columbia Irving Institute for Clinical and Translational Research KL2 Mentored Career Development Award (KL2TR001874). H.C. is supported by National Institute of Neurological Disorders and Stroke (R01 NS104076). J.F.B. is supported by the National Institute of Minority Health and Health Disparities (R01 MD008879).
Terman SW, Youngerman BE, Choi H, Burke JF. Antiseizure medication treatment pathways for US Medicare beneficiaries with newly treated epilepsy. Epilepsia. 2022;63:1571–1579. 10.1111/epi.17226
REFERENCES
- 1. Brodie MJ. Antiepileptic drug therapy the story so far. Seizure. 2010;19(10):650–5. 10.1016/j.seizure.2010.10.027 [DOI] [PubMed] [Google Scholar]
- 2. Nevitt S, Sudell M, Weston J, Turdur Smith C, Marson A. Antiepileptic drug monotherapy for epilepsy: a network meta‐analysis of individual participant data. Cochrane Database Syst Rev. 2017;12:CD011412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glauser T, Ben‐Menachem E, Bourgeois B, Cnaan A, Guerreiro C, Kälviäinen R, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54(3):551–63. [DOI] [PubMed] [Google Scholar]
- 4. Kanner AM, Ashman E, Gloss D, Harden C, Bourgeois B, Bautista JF, et al. Practice guideline update summary: efficacy and tolerability of the new antiepileptic drugs I: treatment of new‐onset epilepsy: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2018;91(2):74–81. [DOI] [PubMed] [Google Scholar]
- 5. Chi VUL, Piccenna L, Kwan P, O'Brien T. New‐onset epilepsy in the elderly. Br J Clin Pharmacol. 2018;84:2208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology. 2004;62(5, Suppl 2):S24–9. [DOI] [PubMed] [Google Scholar]
- 7. Loring DW, Marino S, Meador KJ. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol Rev. 2007;17:413–25. [DOI] [PubMed] [Google Scholar]
- 8. Lezaic N, Gore G, Josephson CB, Wiebe S, Jetté N, Keezer MR. The medical treatment of epilepsy in the elderly: a systematic review and meta‐analysis. Epilepsia. 2019;60(7):1325–40. [DOI] [PubMed] [Google Scholar]
- 9. Lattanzi S, Trinka E, Del Giovane C, Nardone R, Silvestrini M, Brigo F. Antiepileptic drug monotherapy for epilepsy in the elderly: a systematic review and network meta‐analysis. Epilepsia. 2019;60(11):2245–54. [DOI] [PubMed] [Google Scholar]
- 10. Martin RC, Faught E, Szaflarski JP, Richman J, Funkhouser E, Piper K, et al. What does the U.S. Medicare administrative claims database tell us about initial antiepileptic drug treatment for older adults with new‐onset epilepsy? Epilepsia. 2017;58(4):548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30‐year longitudinal cohort study. JAMA Neurol. 2018;75(3):279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Lott LB, Burke JF, Kerber KA, Skolarus LE, Callaghan BC. Medicare part D payments for neurologist‐prescribed drugs. Neurology. 2016;86(16):1491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. What's Medicare? [Internet]. US Centers for Medicare and Medicaid Services. https://www.medicare.gov/what‐medicare‐covers/your‐medicare‐coverage‐choices/whats‐medicare. Accessed 21 Mar 2022.
- 14. Terman SW, Burke JF, Kerr WT, Marcum ZA, Wang L. Antiseizure medication adherence trajectories in Medicare beneficiaries with newly treated epilepsy. Epilepsia. 2021;62(11):2778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moura LMVR, Smith JR, Blacker D, Vogeli C, Schwamm LH, Cole AJ, et al. Epilepsy among elderly Medicare beneficiaries. Med Care. 2019;57(4):318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jette N, Reid AY, Quan H, Hill MD, Wiebe S. How accurate is ICD coding for epilepsy? Epilepsia. 2010;51(1):62–9. [DOI] [PubMed] [Google Scholar]
- 17. Mbizvo GK, Bennett KH, Schnier C, Simpson CR, Duncan SE, Chin RFM. The accuracy of using administrative healthcare data to identify epilepsy cases: a systematic review of validation studies. Epilepsia. 2019;61:1319–35. [DOI] [PubMed] [Google Scholar]
- 18. Holden EW, Grossman E, Nguyen NT, Gunter MJ, Grebosky B, Von WA, et al. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag. 2005;8(1):1–14. [DOI] [PubMed] [Google Scholar]
- 19. Hripcsak G, Ryan PB, Duke JD, Shah NH, Park RW, Huser V, et al. Characterizing treatment pathways at scale using the OHDSI network. Proc Natl Acad Sci U S A. 2016;113(27):7329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zipcode to carrier locality file [Internet]. Centers for Medicare and Medicaid Services. https://www.cms.gov/medicare/medicare‐fee‐for‐service‐payment/feeschedulegeninfo. Accessed 21 Mar 2022.
- 21. Census regions and divisions of the United States [Internet]. US Department of Commerce Economics and Statistics Administration, US Census Bureau. https://www2.census.gov/geo/pdfs/maps‐data/maps/reference/us_regdiv.pdf. Accessed 21 Mar 2022.
- 22. Smith JR, Jones FJS, Fureman BE, Buchhalter JR, Herman ST, Ayub N, et al. Accuracy of ICD‐10‐CM claims‐based definitions for epilepsy and seizure type. Epilepsy Res. 2020;166:106414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. [DOI] [PubMed] [Google Scholar]
- 24. Comorbidity SAS macro [Internet]. National Cancer Institute. 2014. https://healthcaredelivery.cancer.gov/seermedicare/considerations/macro‐2014.html. Accessed 21 Mar 2022.
- 25. Using appropriate price indices for analyses of health care expenditures or income across multiple years [Internet]. Agency for Healthcare Research and Quality. 2021. https://meps.ahrq.gov/about_meps/Price_Index.shtml. Accessed 21 Mar 2022.
- 26. Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res. 2016;53(1):175–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Terman S, Kerr T, Aubert C, Hill C, Marcum Z, Burke J. Adherence to antiseizure vs other medications among US Medicare beneficiaries with and without epilepsy. Neurology. 2022;98(4):e427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Josephson CB, Engbers JDT, Jette N, Patten SB, Singh S, Sajobi TT, et al. Prediction tools for psychiatric adverse effects after levetiracetam prescription. JAMA Neurol. 2019;76(4):440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brodie MJ, Yuen AWC. Lamotrigine substitution study: evidence for synergism with sodium valproate? Epilepsy Res. 1997;26(3):423–32. [DOI] [PubMed] [Google Scholar]
- 30. Brodie MJ. Sodium channel blockers in the treatment of epilepsy. CNS Drugs. 2017;31(7):527–34. [DOI] [PubMed] [Google Scholar]
- 31. French JA, Kanner AM, Bautista J, Abou‐Khalil B, Browne T, Harden CL, et al. Efficacy and tolerability of the new antiepileptic drugs, I: treatment of new‐onset epilepsy: report of the TTA and QSS Subcommittees of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2004;45(5):410–23. [DOI] [PubMed] [Google Scholar]
- 32. Percentage of people covered by Medicare in the United States from 1990 to 2020 [Internet]. Statista. 2021. https://www.statista.com/statistics/200962/percentage‐of‐americans‐covered‐by‐medicare/. Accessed 21 Mar 2022.
- 33. Jette N, Beghi E, Hesdorffer D, Moshé SL, Zuberi SM, Medina MT, et al. ICD coding for epilepsy: past, present, and future—a report by the International League Against Epilepsy Task Force on ICD codes in epilepsy. Epilepsia. 2015;56(3):348–55. [DOI] [PubMed] [Google Scholar]
- 34. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. A practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Table S1‐S2
Data Availability Statement
All datasets are available to purchase at https://www.resdac.org/. Aggregated deidentified data may be shared upon request.


