Abstract
Insensitivity to standard clinical interventions, including chemotherapy, radiotherapy and tyrosine kinase inhibitor (TKI) treatment, remains a substantial hindrance towards improving the prognosis of patients with non-small cell lung cancer (NSCLC). The molecular mechanism of therapeutic resistance remains poorly understood. The TNF-like weak inducer of apoptosis (TWEAK)-FGF-inducible 14 (Fn14) signaling axis is known to promote cancer cell survival via NF-κB activation and the up-regulation of pro-survival Bcl-2 family members. Here, a role was determined for TWEAK-Fn14 pro-survival signaling in NSCLC through the up-regulation of myeloid cell leukemia sequence 1 (Mcl-1). Mcl-1 expression significantly correlated with Fn14 expression, advanced NSCLC tumor stage, and poor patient prognosis in human primary NSCLC tumors. TWEAK stimulation of NSCLC cells induced NF-κB-dependent Mcl-1 protein expression and conferred Mcl-1-dependent chemo- and radio-resistance. Depletion of Mcl-1 via siRNA or pharmacological inhibition of Mcl-1, using EU-5148, sensitized TWEAK-treated NSCLC cells to cisplatin- or radiation-mediated inhibition of cell survival. Moreover, EU-5148 inhibited cell survival across a panel of NSCLC cell lines. In contrast, inhibition of Bcl-2/Bcl-xL function had minimal effect on suppressing TWEAK-induced cell survival. Collectively, these results position TWEAK-Fn14 signaling through Mcl-1 as a significant mechanism for NSCLC tumor cell survival, and open new therapeutic avenues to abrogate the high mortality rate seen in NSCLC.
Implications
The TWEAK-Fn14 signaling axis enhances lung cancer cell survival and therapeutic resistance through Mcl-1, positioning both TWEAK-Fn14 and Mcl-1 as therapeutic opportunities in lung cancer.
Introduction
Lung cancer is the leading cause of cancer-related mortality in the USA and throughout the world, with a five-year survival rate for advanced, non-small cell lung cancer (NSCLC), the most common class of lung cancer, below 10%, in part due to intrinsic and acquired resistance to standard therapeutics (1). While targeted therapies have shown promise in small subsets of patients, the majority of lung cancer patients rely on platinum-derived chemotherapeutics and radiation therapy in the absence of more effective targeted therapeutics. Acquired resistance to these treatments remains a significant barrier to reducing mortality in NSCLC patients (2, 3). A deeper understanding of the molecular events leading to therapeutic resistance would identify novel therapeutic targets to improve patient prognosis in advanced NSCLC.
The tumor necrosis factor-like weak inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14 (Fn14; TNFRSF12a) signaling axis has been implicated in a number of solid tumor types and can affect tumor cell proliferation, apoptosis, cell invasion, and cell survival (4). In NSCLC, Fn14 is over-expressed in primary tumors, correlated with activated EGFR, and promoted tumor cell migration and invasion (5). In glioblastoma (GB), TWEAK exposure resulted in enhanced tumor cell invasion through Rac1 and NF-κB activation (6). In addition, TWEAK-Fn14 signaling promoted GB cell survival, primarily through Akt2 phosphorylation, NF-κB activation, and up-regulation of Bcl-2 family members such as Bcl-xL and Bcl-w (7, 8). The role and mechanism(s) of TWEAK-mediated tumor cell survival in NSCLC has not been described.
Pro-survival members of the Bcl-2 family, including Bcl-2, Bcl-xL, Bcl-w, and Mcl-1, are elevated in numerous cancer types and contribute to cancer cell survival and resistance to therapy, largely through direct inhibition of pro-apoptotic Bcl-2 family members (9). Mcl-1 is a mitochondria-associated pro-survival Bcl-2 family member first characterized as a potent, short-term promoter of cell survival during myeloid cell differentiation (10). Mcl-1 is often found to be over-expressed in NSCLC lines compared to normal lung and correlated with poor patient prognosis (11, 12). Mcl-1 binds pro-apoptotic Bcl-2 family members Noxa, Bak, and Bax, thus maintaining their inactive monomeric state and limiting apoptotic signaling, especially in NSCLC lines with high expression of Mcl-1 (13). Further, EGF/ERK signaling induced Mcl-1 and protected NSCLC cells against TKI and chemotherapeutic-induced cell death, with the depletion of Mcl-1 conferring increased sensitization to radiation and chemotherapeutic insult (14). Mcl-1 has been additionally implicated in PI3K/Akt pro-survival signaling in NSCLC; Akt2 knockdown induces Mcl-1 cleavage and mitochondrial-driven cell death (15), and PI3K inhibition leads to decreased Mcl-1 in EGFR mutant lines (16). In an in vivo model of NSCLC driven by c-Myc over-expression and mutant KRAS, Mcl-1 up-regulation was found to be necessary for evasion of apoptosis (17). Thus, Mcl-1 may play a critical role in NSCLC cell survival through antagonizing apoptotic signaling, and could be a novel therapeutic target towards improved efficacy of cytotoxic therapies.
Here, we show that TWEAK-Fn14 pro-survival signaling axis in NSCLC is dependent on Mcl-1. In primary NSCLC tumors, Mcl-1 protein expression was observed in the majority of adenocarcinoma and squamous cell carcinoma specimens. Gene expression of Mcl-1 correlated with higher NSCLC tumor stage and with poor patient outcome. Moreover, the protein and mRNA levels of Mcl-1 and Fn14 were significantly correlated in primary NSCLC tumors. We demonstrate that TWEAK stimulates Mcl-1 expression via NF-κB activity. In addition, we show that TWEAK confers protection against radiation- and cisplatin-induced cell death. Depletion of Mcl-1 protein expression by small inhibitory RNA impaired colony formation in vitro and TWEAK-induced cell survival. Similarly, EU-5148, a pharmacological inhibitor of Mcl-1, decreased cell viability across a panel of NSCLC cell lines and reduced TWEAK-induced cell survival. These data suggest a role for both the TWEAK-Fn14 signaling axis and Mcl-1 as therapeutic targets for NSCLC.
Materials and Methods
Cell culture conditions
Human lung adenocarcinoma cell lines H1975 and H2073 (ATCC, Manassas, VA) were maintained in RPMI 1640 media (Invitrogen, Carlsbad, CA) plus 10% heat-inactivated fetal bovine serum (FBS) in a 37°C, 5% CO2 atmosphere. SU-DHL10 (18), Bcl-2 1863 and Mcl-1 1780 cell lines (19) were maintained in RPMI 1640 plus 10% FBS with 50 µM β-mercaptoethanol added to 1863 and 1780 cells. In all assays treated with TWEAK, cells were cultured in reduced serum (0.5% FBS) for 16 hours prior to stimulation with TWEAK at 100 ng/mL in RPMI + 0.1% bovine serum albumin (BSA) for the indicated times.
Immunohistochemistry
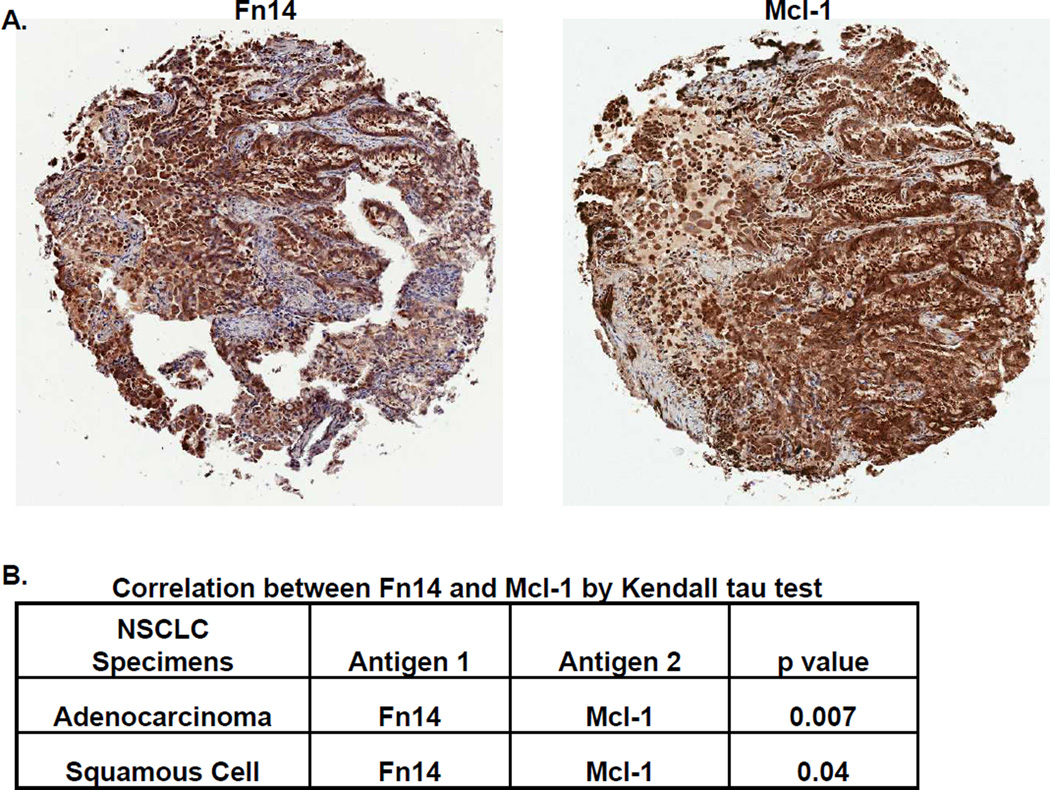
Protein expression by IHC was performed on a tissue microarray as previously described (5), and IHC analysis for Fn14 has been previously described in our lab (5). Mcl-1 staining was performed using an antibody specific for the long form of Mcl-1 (Santa Cruz Biotechnology, Dallas, TX). A scoring system for each chromophore comprised of staining intensity and extensiveness captured the outcome: 0, negative; 1, weak; 2 moderate; 3, strong. A two-sided Kendall's tau test was carried out on scores of Mcl-1 and Fn14 for samples in which both were evaluated and scored.
Antibodies, Reagents, and Immunoblotting
Mcl-1, Bcl-2, Bcl-xL, phospho-p65 (Serine residue 536), Bak, GAPDH, and cleaved-PARP antibodies were obtained from Cell Signaling Technology Inc. (Beverly, MA), and α-tubulin antibody was obtained from Millipore (San Diego, CA). Human recombinant TWEAK was purchased from PeproTech (Rock Hill, NJ), and cisplatin was obtained from TZS Chemical via BIOTANG Inc. (Waltham, MA). The Mcl-1-specific inhibitor EU-5148 (20) was kindly provided by Eutropics Pharmaceuticals (Cambridge, MA). The chemical scaffold of EU-5148 is shown in Supplemental Figure 1 and fully described at the following hyperlink: http://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012122370&recNum=2&office=&queryString=FP%3A%28Eutropics%29&prevFilter=&sortOption=Pub+Date+Desc&maxRec=7. ABT-737 was obtained from Selleck Chemicals (Houston, TX). Immunoblot analysis was performed as previously described (5, 8).
Quantitative, real-time PCR (qPCR)
mRNA expression was determined by qPCR as previously described (21). Briefly, total RNA was extracted from cell lines using the mirVana isolation kit (Ambion, Austin, TX) according to the manufacturer’s directions. cDNA was synthesized from total RNA using SuperScript III First-Strand Synthesis SuperMix (Life Technology, Grand Island, NY) according to the manufacturer’s protocol. Quantitative RT-PCR analyses of Mcl-1 (forward: 5’-GGACTGGCTAGTTAAACAAAGAGG-3’; reverse: 5’-CTTATTAGATATGCCAAACCAGCTC-3’), Bcl-xL (BCL2L1) (forward: 5’-GCTGAGTTACCGGCATCC-3’; reverse: 5’-TTCTGAAGGGAGAGAAAGAGATTC-3’) and histone H3.3 (forward: 5’-CCACTAACTTCTGATTCGC-3’; reverse: 5’-GCGTGCTAGCTGGATGTCTT-3’) were carried out in triplicate in a 384-well plate using a LightCycler 480 (Roche Applied Sciences, Indianapolis, IN) and analyzed as previously described (22).
Expression Plasmids and Transfection
Cytomegalovirus plasmid backbones (pCMV) containing genes for human wild type IκBα or IκBα with serine-to-alanine mutations at residues 32 and 36 were purchased from Addgene (Cambridge, MA) and transfected into the human adenocarcinoma cell lines using the Effectine Transfection Reagent (Qiagen, Valencia, CA) kit according to the manufacturer’s protocol.
Small-interfering RNA preparation and transfection
Small interfering RNA (siRNA) oligonucleotides specific for GL2 Luciferase were previously described (23). Validated siRNA sequences for Mcl-1 full-length transcripts (Mcl-1-1 and Mcl-1-2 target oligo sequences: 5’-CCCGCCGAATTCATTAATTTA-3’, 5’-CCCTAGCAACCTAGCCAGAAA-3’, respectively) and Bcl-xL sequence: 5’-CTGCTTGGGATAAAGATGCAA-3’ were purchased from Qiagen. Transient siRNA transfection was carried out as previously described (23). All siRNA transfections were done at 20 nM siRNA using Lipofectamine RNAi MAX reagent (Invitrogen) and no cytotoxicity were observed 24 hours post-transfection. Maximum inhibition of protein levels was achieved approximately 72 hours post-transfection.
Clonogenic assay
Observations of colony forming capacity following cytotoxic insult were performed as previously described (24). Briefly, cells were transfected with either luciferase or Mcl-1 siRNA, or treated with EU-5148 at 7.5µM for 24 hours, followed by serum-reduction (0.5% FBS) for 16 hours prior to the addition of TWEAK for 24 hours. For cisplatin treatment, cells were pre-treated with TWEAK for 4 hours prior to the addition of cisplatin (1 µM) for 20 hours. For radiation treatment, cells were pre-treated with TWEAK and incubated for 24 hours before exposure to 2Gy ionizing irradiation using an RS-200 (Rad Source, Suwanee, GA). Cells were then trypsinized, quantified, and equally dispersed in triplicate in 6-well cell culture dishes at 250 cells per well. Plates were incubated until colonies reached an approximate size of 50 cells (1–2 weeks) before being fixed briefly in a 10% (v/v) methanol 10% (v/v) glacial acetic acid solution, stained with a 0.5% (w/v) crystal violet solution and washed with deionized water. Apparent colonies were counted. All cell lines and treatments were run in triplicate. Statistical significance, defined as a p value < 0.05, was determined by ANOVA analysis with Bonferroni posttests in Graphpad Prism.
Immunoprecipitation
For immunoprecipitation, cells were treated with TWEAK, 7.5µM EU-5148, or TWEAK and EU-5148 simultaneously. After 24 hours of treatment, cells were lysed on ice for 10 min in a buffer containing 10 mM Tris-HCl (pH 7.4), 0.5% Nonidet P-40, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, 2 mM sodium orthovanadate, 20 mM sodium fluoride, 10 µg/ml aprotinin, and 10 µg/ml leupeptin. Equivalent amounts of protein (500 µg) were pre-cleared and immunoprecipitated from each lysates using Bak antibody as indicated or a control isotype-matched antibody and then washed with lysis buffer followed by TX-100 buffer (10 mM HEPES (pH 7.4), 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 20 mM sodium fluoride, and 0.5% Triton X-100). Immunoprecipitated samples were then reconstituted in 2x SDS buffer containing protease and phosphatase inhibitors and immunoblotted with the indicated antibodies as described.
PrestoBlue Assay
Cell killing was measured by PrestoBlue cell viability reagent after incubating cells with compound for 48 hours. EU-5148 and DMSO were diluted in serum-free RPMI 1640 media and dispensed to a cell culture-treated 384-well plate (Griener Bio-One). Cells in culture were counted and centrifuged, then resuspended in RPMI 1640, 10% FBS, 1% Penicillin-streptomycin. Cells were added to the plate containing drug dilutions (5,000 cells/well) and incubated at 37°C for 48 hours. PrestoBlue cell viability reagent (Invitrogen) was added to the plate and fluorescence was measured after 1 hour at excitation/emission 535/595 nm. Cell killing curves were made in GraphPad Prism 5.
Cell Viability Assay
NSCLC cell viability was tested using the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI) according to the manufacturer’s instructions. Cells were treated with EU-5148 for 48 hours prior to luminescence reading performed on a Victor3 1420 Multilabel Counter (PerkinElmer, Waltham, MA). All cell lines and treatments were performed in duplicate.
Results
Mcl-1 is over-expressed in human primary NSCLC tumors and correlates with Fn14
To determine the expression of MCL-1 protein in NSCLC, we utilized a NSCLC TMA as previously described. The protein expression of Mcl-1 was detected in the cytoplasm in the majority of NSCLC tumors (80% of adenocarcinomas and 58% of squamous cell carcinomas demonstrated moderate to strong protein levels of Mcl-1). Since the correlation of Fn14 expression and pro-survival Bcl-2 family members has been previously reported, we next examined the relationship between these two markers. In both adenocarcinomas and squamous cell carcinomas, the protein levels of Mcl-1 were significantly correlated with the protein levels of Fn14 (Figure 1). Publically available gene expression data (www.genesapiens.org) further showed a significant correlation between Mcl-1 and Fn14 mRNA levels in squamous cell lung cancer specimens (Figure S2). At the gene expression level (Bild Lung data set (25), www.oncomine.org), mRNA levels of Mcl-1 significantly correlate with increasing stage of lung adenocarcinomas (Figure S3A) and with patient mortality at one year (Figure S3B). Thus, Mcl-1 is highly expressed in primary NSCLC tumors, correlates with Fn14 expression, and is associated with poor patient prognosis.
Figure 1. Mcl-1 expression in human NSCLC specimens correlates with Fn14 expression.
(A) Mcl-1 and Fn14 staining on representative samples from the same patient with lung adenocarcinoma (5× objective, Aperio GL Scanner). Tumor-cell specific Fn14 and Mcl-1 staining in each of the tumor punches was scored by a board-certified pathologist; a score of zero indicates staining level equal to adjacent non-tumor cells. A non-zero score indicates increased staining (1= minimum, 2= moderate, 3= strong positive). (B) A total of 290 samples were scored for Mcl-1 and Fn14 expression and the correlation between the two stains was analyzed using Kendall’s tau test.
TWEAK stimulation induces Mcl-1 and other Bcl-2 family member expression through activation of NF-κB
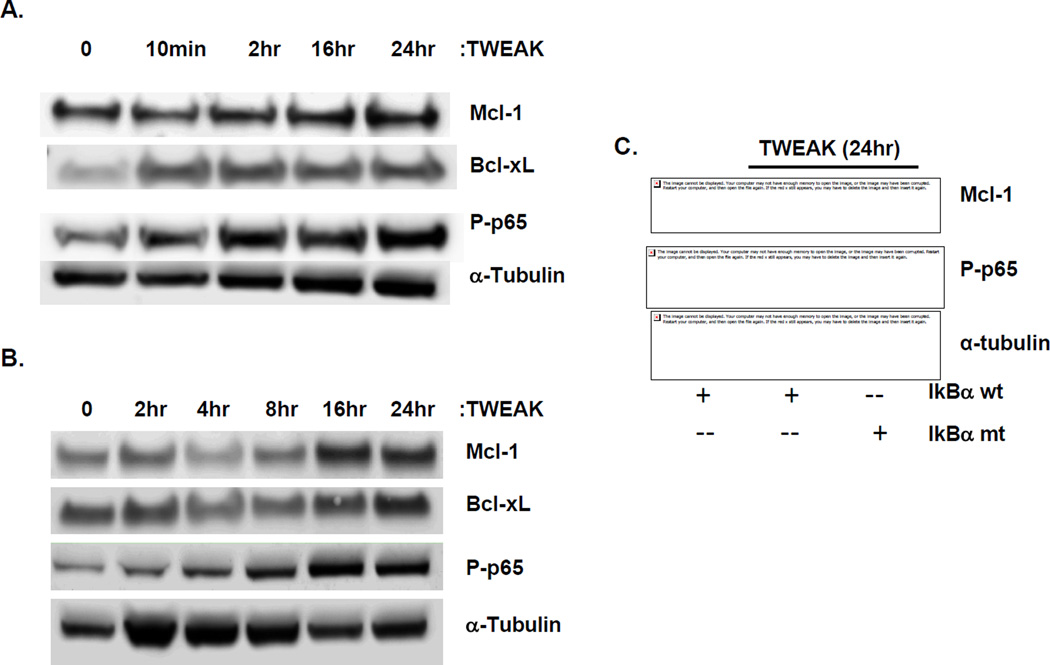
TWEAK promotes cancer cell survival through phosphorylation of the p65 subunit of NF-κB, leading to increased expression of pro-survival Bcl-2 family members Bcl-xL and Bcl-w (8). We therefore investigated whether TWEAK stimulation induces pro-survival Bcl-2 members in NSCLC. In the adenocarcinoma cell lines H1975 and H2073, TWEAK treatment led to the clear induction of phosphorylated p65 (P-p65), with concomitant incremental increases in protein expression of Mcl-1 and Bcl-xL over time. (Figure 2A and B) The protein expression of Bcl-2 was also up-regulated following TWEAK exposure in H1975, but was not expressed in H2073 (data not shown). To confirm TWEAK-induced expression of Bcl-2 family members, we measured mRNA expression by qPCR. TWEAK treatment induced mRNA levels of Mcl-1 and Bcl-xL in both H1975 and H2073 cells as early as 30 minutes post-TWEAK with maximal expression ~6 hours (10-fold increase in H1975 and 50-fold increase in H2073) (Figure S4A and B).
Figure 2. TWEAK induces Mcl-1 in NSCLC cell lines in an NF-κB-dependent manner.
Total cell lysates were prepared from serum-reduced (A) H1975 and (B) H2073 cell lines treated with TWEAK for the indicated times and immunoblotted with the indicated antibodies: Mcl-1, Bcl-xL, and phosphorylated-p65 (Ser536). Tubulin was used as a loading control. (C) Serum-reduced H2073 cells transfected ± IkBα mutant were treated with TWEAK for 24 hours. Cells were harvested, total cell lysates were prepared and immunoblotted with the indicated antibodies to both Mcl-1 and phospho-p65. All blots were run in duplicate and tubulin was used as a loading control.
To determine whether TWEAK-induced Mcl-1 expression is dependent on NF-κB activation, H2073 cells were transfected with wild-type IκBα (IκBα-wt), or a mutated IκBα super-repressor (IκBα-mt) expressing plasmid, and levels of Mcl-1 following TWEAK exposure were assessed. The IκBα-mt is incapable of being phosphorylated and thus sequesters NF-κB in the cytoplasm. Mcl-1 protein expression increased with TWEAK treatment in IκBα-wt-expressing cells, but was inhibited in the presence of the IκBα-mt expression, in correlation with reduced p65 phosphorylation (Figure 2C). These data suggest that NF-κB activation by TWEAK is necessary for the induction of Mcl-1 in NSCLC.
TWEAK-induced NSCLC cell survival is dependent on Mcl-1 expression
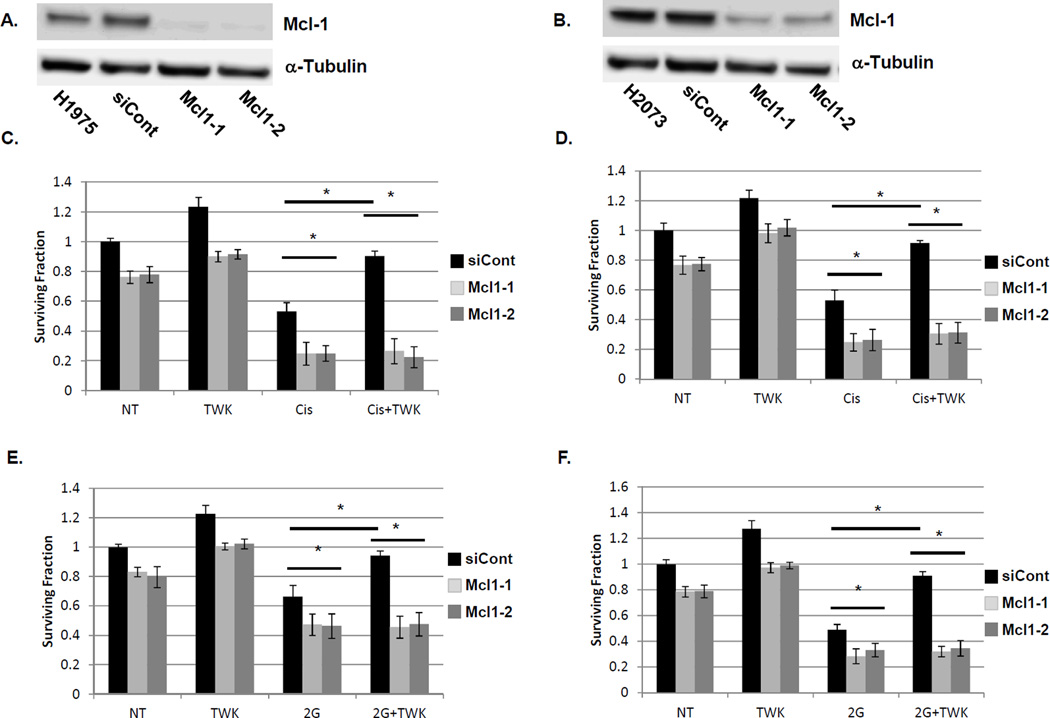
We next sought to characterize the functional role of Mcl-1 in TWEAK-induced tumor cell survival. The protein expression of Mcl-1 was depleted by targeted siRNA constructs in both H1975 and H2073 cells (Figure 3A and 3B, respectively). Cell survival was assessed by colony-formation assay. In both H1975 and H2073, exposure to ionizing radiation or cisplatin significantly reduced NSCLC cell survival (Figure 3C-F). siRNA mediated depletion of Mcl-1 significantly enhanced sensitivity to either cisplatin or radiation compared to control cells expressing non-targeting siRNA oligonucleotides. TWEAK pre-treatment significantly attenuated the effects of either cisplatin or radiation, back to untreated surviving fractions. Depletion of Mcl-1 via siRNA oligonucleotides completely abrogated the protective effects observed with TWEAK pre-treatment. However, depletion of Bcl-xL could not fully rescue the TWEAK induced cell survival as seen with Mcl-1 depletion (Figure S5A-B). Thus, TWEAK exposure may protect NSCLC cells from DNA-damaging therapies such as radiation and cisplatin; and this protective phenotype appears to be dependent on Mcl-1 function.
Figure 3. TWEAK-induced NSCLC cell survival is dependent on Mcl-1 expression.
H1975 (A) and H2073 (B) cells were transfected with luciferase (siCont) or siRNAs targeting Mcl-1. Total lysates were collected 72 hours post-transfection and immunoblotted for Mcl-1 and alpha-tubulin. H1975 (C and E) and H2073 (D and F) cells transfected with control or siRNA constructs targeting Mcl-1 were exposed to 1 µM cisplatin for 24 hours (C and D) or 2Gy ionizing radiation (E and F) ± pre-incubation with TWEAK (100 ng/mL). Cells were sparsely seeded into 6-well dishes and allowed to grow for 7 days prior to staining with crystal violet and colony counting. A colony was defined as containing at least 50 cells. Bars represent average of three independent wells ± standard error with the non-treated (first bar) set to 1. * represents a p value < 0.05 by ANOVA with Bonferroni posttest.
To confirm the effects of TWEAK signaling and the role of Mcl-1 in NSCLC cell survival, we assessed apoptosis through the induction of cleaved-PARP in H1975 cells exposed to cisplatin and radiation. Figure 4 demonstrates that exposure to cisplatin (Fig. 4A) or radiation (Fig. 4B) induces protein expression of cleaved-PARP over time (lanes 5 and 9 compared to 1). Pretreatment with TWEAK completely abrogates the induction of cleaved-PARP (lanes 6 and 10 compared to lanes 5 and 9). The depletion of Mcl-1 through siRNA results in enhanced induction of cleaved-PARP compared to cisplatin or radiation alone, an enhanced sensitivity that was not affected by TWEAK exposure.
Figure 4. Depletion of Mcl-1 abrogates TWEAK-induced protection from cell death induced by DNA damage.
H1975 cells were transfected with either siRNA targeting luciferase (control) or Mcl-1. Cells were exposed to 5uM cisplatin (A) or 8Gy radiation (B) for 0, 4 or 24 hours ± pre-incubation with TWEAK (100 ng/mL). Total cell lysates were prepared and immunblotted for cleaved-PARP (cPARP) and GAPDH as a loading control. All blots were run in duplicate.
Mcl-1 pharmacological inhibitor EU-5148 decreases NSCLC cell survival
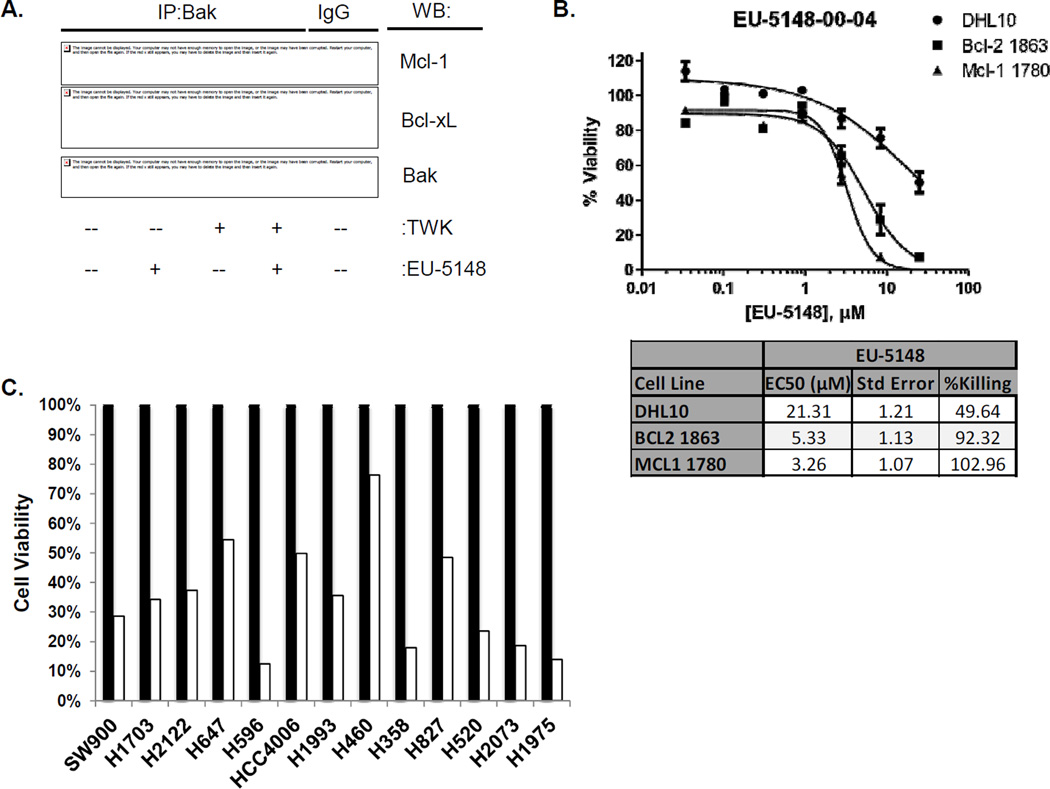
While inhibitors of Bcl-2/Bcl-xL have been well described, specific inhibitors of Mcl-1 are now being investigated (26–28). We explored the use of a Mcl-1-specific pharmacological inhibitor designated EU-5148 (Figure S1) as an antagonist of NSCLC cell survival. Figure 5A demonstrates that exposure to EU-5148 specifically disrupts the protein-protein interaction of Mcl-1 and Bak, while not affecting the interaction of Bcl-xL and Bak. H1975 cells were treated with EU-5148 in the presence or absence of TWEAK. Cells were immunoprecipitated with anti-Bak antibodies and immunoblotted for Mcl-1, Bcl-xL and Bak. Exposure to EU-5148 suppressed the protein interaction between Mcl-1 and Bak with or without TWEAK exposure. Conversely, exposure to EU5148 had no effect on the protein interaction of Bcl-xL and Bak. To further show Mcl-1 specificity for EU-5148, we employed cell lines deficient of Bax and Bim (DHL10) (18) or driven by Mcl-1 (Mcl-1 1780) or Bcl-2 (Bcl-2 1863). Figure 5B shows that cells driven by Mcl-1 are most sensitive to EU-5148 (EC50 = 3.26 µM), while cells deficient for Bax/Bim were less sensitive to EU-5148 (EC50 = 21.31 µM). Furthermore, an ELISA-based competitive displacement assay demonstrated that EU-5148 was ~3.5-fold more disruptive of a Mcl-1-Bim protein interaction compared to a Bcl-xL-Bim protein interaction (Figure S6).
Figure 5. Pharmacologic inhibition of Mcl-1 inhibits NSCLC cell growth.
(A) H1975 cells were grown in the presence or absence of TWEAK (100 ng/mL) and EU-5148 (10µM). Cells were lysed and immunoprecipitated with anti-Bak antibodies. Protein expression Mcl-1, Bcl-xL and Bak after immunoprecipitation were resolved by immunoblot analysis. (B) Cell viability of DHL10, Bcl-2 1863 and Mcl-1 1780 cells was assessed by PrestoBlue assay. Cells were exposed to the indicated concentrations of EU-5148 in DMSO for 48 hours. Cell killing curves and EC-50 values were generated from triplicate runs in GraphPad Prism 5. (C) A panel of NSCLC cell lines was exposed to vehicle or 10 µM EU-5148 for 48 hours. Cell growth was assessed by Cell-Titer Glo assay. Bars represent the average of two wells with the untreated set to 100%.
In a panel of NSCLC cell lines, EU-5148 significantly diminishes cell viability 48 hours post-treatment compared to non-treated cells (Figure 5C). Cell viability was reduced between 25–87% across 13 NSCLC cell lines with 11 of the 13 lines showing > 50% reduction in cell viability.
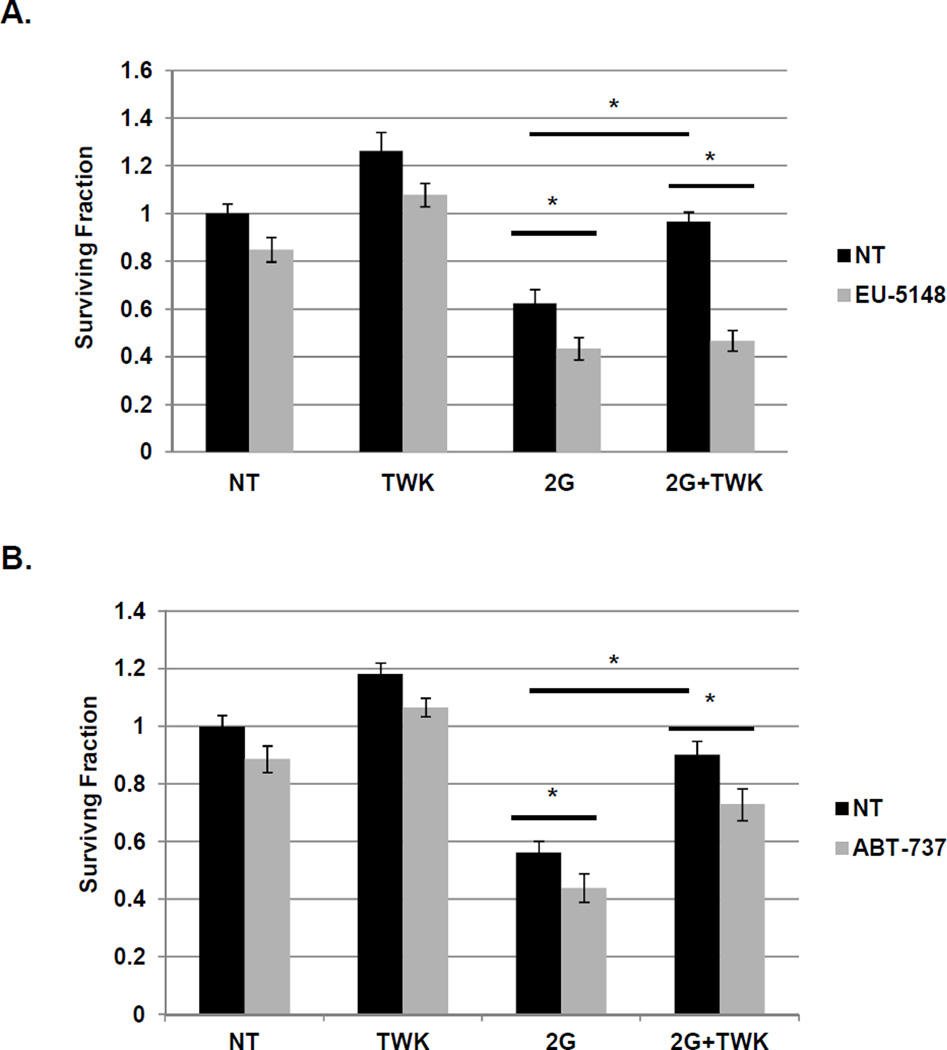
Lastly, we sought to assess whether EU-5148 could suppress TWEAK-induced NSCLC cell survival. Exposure of H1975 cells to radiation significantly reduces cell survival, whereas, exposure to the Mcl-1 inhibitor EU-5418 further enhances sensitivity to radiation (Figure 6A). Pre-treatment with TWEAK completely abrogates the reduction in cell survival induced by radiation exposure, but Mcl-1 inhibition restores radiation sensitivity, even with TWEAK exposure. Similar effects are observed with EU-5418 exposure in H1975 cells exposed to cisplatin and H2073 cells exposed to radiation or cisplatin (Figure S7). While Mcl-1 inhibition abrogated TWEAK-induced cancer cell survival, exposure to ABT-737, a potent inhibitor of Bcl-2 and Bcl-xL, had a lesser effect on TWEAK-induced cell survival (Fig. 6B). Although ABT-737 did sensitize H1975 cells to radiation, the TWEAK-induced cell rescue was only minimally significant, suggesting a critical role for Mcl-1 in the TWEAK-induced cell survival effects in NSCLC.
Figure 6. Pharmacologic inhibition of Mcl-1 abrogates TWEAK-mediated cell survival.
H1975 (A and B) cells were pre-incubated with TWEAK (100 ng/mL), (A) EU-5148 (10 µM), (B) ABT-737 (10 µM) or both drug and TWEAK prior to exposure to 2Gy ionizing radiation. Cells were sparsely seeded (125 cells) into 6-well dishes and allowed to grow for 7 days prior to staining with crystal violet and colony counting. A colony was defined as containing at least 50 cells. Bars represent average of three independent wells ± standard error with the non-treated (first bar) set to 1. * represents a p value < 0.05 by ANOVA with Bonferroni posttest.
Discussion
Pro-survival members of the Bcl-2 family are well-characterized antagonists of apoptotic signaling, mediating cell survival downstream of a variety of cytotoxic insults (29). Specifically, Mcl-1 plays an important pro-survival role in NSCLC by limiting cytotoxicity of chemo- or radiation-therapy and TKI treatments (14), in addition to showing a significant role in evasion of apoptosis in a variety of solid tumors (17, 30). In this study, we demonstrated that Mcl-1 is regulated through the TWEAK/Fn14 signaling axis and is necessary to promote TWEAK-mediated NSCLC cell survival. Protein expression of Fn14 and Mcl-1 are significantly correlated in primary NSCLC tumors, and treatment with TWEAK induces expression of Mcl-1 through activation of NF-κB. Inhibition of Mcl-1 expression promotes chemo- and radio-sensitivity and abrogates TWEAK-induced tumor cell survival. This work positions both the TWEAK-Fn14 pathway and Mcl-1 as potential therapeutic interventions targeting lung tumor evasion of apoptosis.
To date, the functional role of Fn14 in lung cancer remains poorly understood. We previously reported over-expression of Fn14 in primary NSCLC (5) and showed correlation of Fn14 with activated EGFR in NSCLC and a positive association with cell motility and metastasis. Activation of Fn14 signaling through TWEAK or receptor over-expression leads to enhanced tumor cell migration/invasion, angiogenesis, and tumor cell survival (4). In GB tumor cells, TWEAK stimulation promotes cell survival through Akt2 (7) and the NF-κB-dependent up-regulation of pro-survival Bcl-2 family members (8). Our data supports a TWEAK-Fn14 induced NSCLC cell survival by induction of pro-survival Bcl-2 family member, specifically Mcl-1, in a NF-κB-dependent manner. TWEAK exposure induced both protein and mRNA expression of Mcl-1 and Bcl-xL in NSCLC cell lines. We further demonstrated that protein and gene expressions of Fn14 and Mcl-1 were significantly correlated in primary lung tumor specimens. Thus, the TWEAK-Fn14 signaling axis and the pro-survival Mcl-1 gene may cooperate in NSCLC cell survival and represent potential therapeutic targets.
Over-expression of pro-survival Bcl-2 family members such as Mcl-1 is a well-characterized event in tumor progression, encouraging cell survival (29). Here, we demonstrate a role for Mcl-1 in NSCLC tumor survival as a critical downstream component of TWEAK/Fn14 signaling. The depletion of Mcl-1 through siRNA or pharmacologic inhibitor (EU-5148) was sufficient to abrogate the protective effects conferred on lung tumor cells by TWEAK/Fn14 signaling, whereas inhibition of Bcl-2 and Bcl-xL had a lesser effect. Despite similarities in transcriptional regulation of Mcl-1 and other pro-survival members of the Bcl-2 family, an increasing body of evidence indicates multiple tumor types have over-dependence on Mcl-1 alone to negate apoptotic signaling (31). For example, IHC staining of Mcl-1 proved to be a better prognostic indicator of ovarian carcinoma progression in a group of patient biopsies compared to Bcl-2 or Bcl-xL (32). Inhibition of Mcl-1 in multiple myeloma by antisense oligonucleotides induced apoptosis, where inhibition of Bcl-2 or Bcl-xL could not mimic this effect (33). Interestingly, resistance to inhibitors of Bcl-2 and Bcl-xL can be achieved through Mcl-1 expression (34). These data suggest non-overlapping roles for the Bcl-2 pro-survival family members in cancer contexts, and position Mcl-1 as a critical regulator of tumor cell survival.
The complex molecular interactions between pro-survival and pro-death members of the Bcl-2 family are known to determine cancer cell survival/apoptosis. A shift in the interactions of pro-survival Bcl-2 members with pro-death members in response to survival stimuli is only beginning to be understood. Lopez et al. showed that DNA damage-induced apoptosis only occurred when Bcl-xL and Mcl-1 were inhibited (35). DNA damage induced a Noxa-Bcl-xL interaction that prompted cytochrome c release only when Mcl-1 was degraded. Bcl-xL and Mcl-1 have been shown to be inhibitors of apoptosis induced by TNF-related apoptosis-inducing ligand (TRAIL), with Noxa playing a critical role in sequestering Bcl-xL and Mcl-1 (36). ER stress or proteasome inhibition could induce a switch from Noxa-Mcl-1 to Noxa-Bcl-xL, most likely through degradation of Mcl-1. Here we demonstrated that EU-5148 disrupts the protein interaction of Mcl-1 and Bak without affecting the Bcl-xL-Bak protein interaction. We further showed that Mcl-1 inhibition fully abrogated TWEAK induced cell survival, while Bcl-xL depletion or Bcl-2/Bcl-xL inhibition only modestly affected TWEAK-induced cell survival. Other TNF ligands have shown preference towards Bcl-xL for cell survival. The suppression of Bcl-xL, but not Mcl-1 or Bcl-2, rendered cells sensitive to TNFα-induced apoptosis (37). These data support a complex relationship between Bcl-2 family interactions downstream of cell survival and apoptotic stimuli.
Inhibition of Mcl-1 has potential for clinical impact in NSCLC and a variety of other tumor types, although specific pharmacological inhibition of Mcl-1 has been elusive. To date several pan-Bcl-2 inhibitors have been developed, such as ABT-737, and inhibit tumor growth and survival across a spectrum of tumor types (29, 38). Though progressing through clinical trials, ABT-737 does not inhibit Mcl-1 and expression of Mcl-1 leads to resistance to pan-Bcl-2 inhibitors (34). Obatoclax and sabutoclax are pan-Bcl-2 inhibitors that do target Mcl-1 and have shown inhibitory effects in a number of tumor cell lines (27, 39). Depletion or degradation of Mcl-1 as a consequence of targeted inhibitors has been recently reviewed (27). Proteasome inhibitors result in Mcl-1 degradation (40), while mTOR inhibition leads to Mcl-1 suppression in mutant kRas-driven colorectal cancer (41). There have been reports more recently of compounds that are Mcl-1-specific inhibitors. UMI-77 is a novel small molecule inhibitor of Mcl-1, which showed anti-tumor activity against pancreatic cancer through disruption of Mcl-1-Bax and Mcl-1-Bak interactions (26). A recent article described a number of hydroxyquinoline-derived compounds with specific affinity for Mcl-1 over Bcl-xL (28). Here we show that EU-5148 specifically disrupts the Mcl-1-Bak protein interaction while not affecting the Bcl-xL-Bak interaction by immunoprecipitation, and demonstrate increased cell killing in cells driven by Mcl-1 compared to cells driven by Bcl-2 or cells deficient in Bax and Bim. EU-5148 decreased cell viability across a panel of NSCLC cell lines. As shown with specific siRNA depletion of Mcl-1, EU-5148 abrogated TWEAK-induced radio- or chemo-resistance in NSCLC cells, an inhibition not observed with ABT-737 exposure. Thus specific inhibitors of Mcl-1 may be more effective in reducing tumor cell survival in contexts such as those involving TWEAK/Fn14 activation. Future work will characterize the use of EU-5148 in an in vivo setting both as a mono-therapy and in combination with standard of care cytotoxic agents.
Mcl-1 may also play a critical role in progenitor/stem cell regulation in normal and tumor cells. A review by Perciavalle and Opferman highlighted the necessity of Mcl-1 for early embryonic development and the survival of multiple cell lineages (30). Tumor progenitor cells are associated with tumor self-renewal and therapeutic resistance (42, 43). Singh et al. recently demonstrated that NSCLC stem-like cells showed higher Mcl-1 expression compared to the main population of cells; and inhibition with obatoclax prevented self-renewal of resistant NSCLC cells (44). Over-expression of Mcl-1 in transgenic mice lead to lymphoma development with a progenitor cell phenotype, as well as lymphoid and myeloid cells highly resistant to a variety of cytotoxic agents (45). Future studies will be aimed at understanding the effect that Mcl-1-specific inhibitors (such as EU-5148) have on tumor progenitor cells, as well as the potential for pathways that induce Mcl-1 (such as TWEAK/Fn14) to affect progenitor cell populations.
In summary, our study showed that the TWEAK-Fn14 signaling axis promotes survival in NSCLC, via NF-κB-dependent induction of Mcl-1. Inhibition of Mcl-1 function enhanced chemo- and radio-sensitivity in NSCLC cells. This work positions the TWEAK-Fn14 signaling axis and Mcl-1 expression as important features in NSCLC cell survival and warrants further investigation into therapeutic avenues inhibiting these pathways towards reducing lung cancer mortality.
Supplementary Material
Acknowledgements
We would like to acknowledge Eutropics Pharmaceuticals for kindly providing the EU-5148 inhibitor and Camille Doykan for technical assistance. This work was supported in whole or in part, by the National Institutes of Health Grant R01 CA130940 (to N.L.T.).
Abbreviations
- NSCLC
Non-small cell lung cancer
- TWEAK
tumor necrosis factor-like weak inducer of apoptosis
- Fn14
fibroblast growth factor-inducible 14
- Mcl-1
myeloid cell leukemia sequence 1
- Bcl-2
B-cell lymphoma 2
Footnotes
The authors declare that this work has not been previously published and have no conflicts of interest to disclose.
References
- 1.Heist RS, Engelman JA. SnapShot: non-small cell lung cancer. Cancer Cell. 2012;21:448 e2. doi: 10.1016/j.ccr.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Chang A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer. 2011;71:3–10. doi: 10.1016/j.lungcan.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Hildebrandt MA, Gu J, Wu X. Pharmacogenomics of platinum-based chemotherapy in NSCLC. Expert Opin Drug Metab Toxicol. 2009;5:745–755. doi: 10.1517/17425250902973711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov. 2008;7:411–425. doi: 10.1038/nrd2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitsett TG, Cheng E, Inge L, Asrani K, Jameson NM, Hostetter G, et al. Elevated expression of Fn14 in non-small cell lung cancer correlates with activated EGFR and promotes tumor cell migration and invasion. Am J Pathol. 2012;181:111–120. doi: 10.1016/j.ajpath.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran NL, McDonough WS, Savitch BA, Fortin SP, Winkles JA, Symons M, et al. Increased fibroblast growth factor-inducible 14 expression levels promote glioma cell invasion via Rac1 and nuclear factor-kappaB and correlate with poor patient outcome. Cancer Res. 2006;66:9535–9542. doi: 10.1158/0008-5472.CAN-06-0418. [DOI] [PubMed] [Google Scholar]
- 7.Fortin SP, Ennis MJ, Savitch BA, Carpentieri D, McDonough WS, Winkles JA, et al. Tumor necrosis factor-like weak inducer of apoptosis stimulation of glioma cell survival is dependent on Akt2 function. Mol Cancer Res. 2009;7:1871–1881. doi: 10.1158/1541-7786.MCR-09-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran NL, McDonough WS, Savitch BA, Sawyer TF, Winkles JA, Berens ME. The tumor necrosis factor-like weak inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14 (Fn14) signaling system regulates glioma cell survival via NFkappaB pathway activation and BCL-XL/BCL-W expression. J Biol Chem. 2005;280:3483–3492. doi: 10.1074/jbc.M409906200. [DOI] [PubMed] [Google Scholar]
- 9.Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414–1424. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borner MM, Brousset P, Pfanner-Meyer B, Bacchi M, Vonlanthen S, Hotz MA, et al. Expression of apoptosis regulatory proteins of the Bcl-2 family and p53 in primary resected non-small-cell lung cancer. Br J Cancer. 1999;79:952–958. doi: 10.1038/sj.bjc.6690152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo L, Zhang T, Liu H, Lv T, Yuan D, Yao Y, et al. MiR-101 and Mcl-1 in non-small-cell lung cancer: expression profile and clinical significance. Med Oncol. 2012;29:1681–1686. doi: 10.1007/s12032-011-0085-8. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Guttikonda S, Roberts L, Uziel T, Semizarov D, Elmore SW, et al. Mcl-1 is critical for survival in a subgroup of non-small-cell lung cancer cell lines. Oncogene. 2011;30:1963–1968. doi: 10.1038/onc.2010.559. [DOI] [PubMed] [Google Scholar]
- 14.Song L, Coppola D, Livingston S, Cress D, Haura EB. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biol Ther. 2005;4:267–276. doi: 10.4161/cbt.4.3.1496. [DOI] [PubMed] [Google Scholar]
- 15.Lee MW, Kim DS, Lee JH, Lee BS, Lee SH, Jung HL, et al. Roles of AKT1 and AKT2 in non-small cell lung cancer cell survival, growth, and migration. Cancer Sci. 2011;102:1822–1828. doi: 10.1111/j.1349-7006.2011.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci U S A. 2009;106:19503–19508. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen TD, Zhu CQ, Jones KD, Yanagawa N, Tsao MS, Bishop JM. Interaction between MYC and MCL1 in the genesis and outcome of non-small-cell lung cancer. Cancer Res. 2011;71:2212–2221. doi: 10.1158/0008-5472.CAN-10-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Ryan JA, Brunelle JK, Letai A. Heightened mitochondrial priming is the basis for apoptotic hypersensitivity of CD4+ CD8+ thymocytes. Proc Natl Acad Sci U S A. 2010;107:12895–12900. doi: 10.1073/pnas.0914878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardone MH. inventor Eutropics Pharmaceuticals Inc , assignee. Compositions and methods useful for treating diseases. 2011 [Google Scholar]
- 21.Fortin Ensign SP, Mathews IT, Eschbacher JM, Loftus JC, Symons MH, Tran NL. The Src homology 3 domain-containing guanine nucleotide exchange factor is overexpressed in high-grade gliomas and promotes tumor necrosis factor-like weak inducer of apoptosis-fibroblast growth factor-inducible 14-induced cell migration and invasion via tumor necrosis factor receptor-associated factor 2. J Biol Chem. 2013;288:21887–21897. doi: 10.1074/jbc.M113.468686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran NL, McDonough WS, Donohue PJ, Winkles JA, Berens TJ, Ross KR, et al. The human Fn14 receptor gene is up-regulated in migrating glioma cells in vitro and overexpressed in advanced glial tumors. Am J Pathol. 2003;162:1313–1321. doi: 10.1016/S0002-9440(10)63927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chuang YY, Tran NL, Rusk N, Nakada M, Berens ME, Symons M. Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res. 2004;64:8271–8275. doi: 10.1158/0008-5472.CAN-04-2097. [DOI] [PubMed] [Google Scholar]
- 24.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 25.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 26.Abulwerdi F, Liao C, Liu M, Azmi AS, Aboukameel A, Mady AS, et al. A Novel Small-Molecule Inhibitor of Mcl-1 Blocks Pancreatic Cancer Growth In vitro and In vivo. Mol Cancer Ther. 2013 doi: 10.1158/1535-7163.MCT-12-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinn BA, Dash R, Azab B, Sarkar S, Das SK, Kumar S, et al. Targeting Mcl-1 for the therapy of cancer. Expert Opin Investig Drugs. 2011;20:1397–1411. doi: 10.1517/13543784.2011.609167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard DJ, Lena R, Bannister T, Blake N, Pierceall WE, Carlson NE, et al. Hydroxyquinoline-derived compounds and analoguing of selective Mcl-1 inhibitors using a functional biomarker. Bioorg Med Chem. 2013;21:6642–6649. doi: 10.1016/j.bmc.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol. 2012;30:3127–3135. doi: 10.1200/JCO.2011.37.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perciavalle RM, Opferman JT. Delving deeper: MCL-1's contributions to normal and cancer biology. Trends Cell Biol. 2013;23:22–29. doi: 10.1016/j.tcb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Placzek WJ, Wei J, Kitada S, Zhai D, Reed JC, Pellecchia M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010;1:e40. doi: 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shigemasa K, Katoh O, Shiroyama Y, Mihara S, Mukai K, Nagai N, et al. Increased MCL-1 expression is associated with poor prognosis in ovarian carcinomas. Jpn J Cancer Res. 2002;93:542–550. doi: 10.1111/j.1349-7006.2002.tb01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JL, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood. 2002;100:194–199. doi: 10.1182/blood.v100.1.194. [DOI] [PubMed] [Google Scholar]
- 34.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Lopez H, Zhang L, George NM, Liu X, Pang X, Evans JJ, et al. Perturbation of the Bcl-2 network and an induced Noxa/Bcl-xL interaction trigger mitochondrial dysfunction after DNA damage. J Biol Chem. 2010;285:15016–15026. doi: 10.1074/jbc.M109.086231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Lopez H, George NM, Liu X, Pang X, Luo X. Selective involvement of BH3-only proteins and differential targets of Noxa in diverse apoptotic pathways. Cell Death Differ. 2011;18:864–873. doi: 10.1038/cdd.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casanelles E, Gozzelino R, Marques-Fernandez F, Iglesias-Guimarais V, Garcia-Belinchon M, Sanchez-Osuna M, et al. NF-kappaB activation fails to protect cells to TNFalpha-induced apoptosis in the absence of Bcl-xL, but not Mcl-1, Bcl-2 or Bcl-w. Biochim Biophys Acta. 2013;1833:1085–1095. doi: 10.1016/j.bbamcr.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Thomas S, Quinn BA, Das SK, Dash R, Emdad L, Dasgupta S, et al. Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther Targets. 2013;17:61–75. doi: 10.1517/14728222.2013.733001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joudeh J, Claxton D. Obatoclax mesylate : pharmacology and potential for therapy of hematological neoplasms. Expert Opin Investig Drugs. 2012;21:363–373. doi: 10.1517/13543784.2012.652302. [DOI] [PubMed] [Google Scholar]
- 40.Fan F, Tonon G, Bashari MH, Vallet S, Antonini E, Goldschmidt H, et al. Targeting Mcl-1 for multiple myeloma (MM) therapy: Drug-induced generation of Mcl-1 fragment Mcl-1 triggers MM cell death via c-Jun upregulation. Cancer Lett. 2013 doi: 10.1016/j.canlet.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 41.Faber AC, Coffee EM, Costa C, Dastur A, Ebi H, Hata AN, et al. mTOR Inhibition Specifically Sensitizes Colorectal Cancers with KRAS or BRAF Mutations to BCL-2/BCL-XL inhibition by Suppressing MCL-1. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-13-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francipane MG, Chandler J, Lagasse E. Cancer Stem Cells: A Moving Target. Curr Pathobiol Rep. 2013;1:111–118. doi: 10.1007/s40139-013-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol. 2013;85:1219–1226. doi: 10.1016/j.bcp.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Singh S, Bora-Singhal N, Kroeger J, Laklai H, Chellappan SP. betaArrestin-1 and Mcl-1 modulate self-renewal growth of cancer stem-like side-population cells in non-small cell lung cancer. PLoS One. 2013;8:e55982. doi: 10.1371/journal.pone.0055982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell KJ, Bath ML, Turner ML, Vandenberg CJ, Bouillet P, Metcalf D, et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood. 2010;116:3197–3207. doi: 10.1182/blood-2010-04-281071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.