Abstract
Cell differentiation requires different pathways to act in concert to produce a specialized cell type. The budding yeast Saccharomyces cerevisiae undergoes filamentous growth in response to nutrient limitation. Differentiation to the filamentous cell type requires multiple signaling pathways, including a mitogen-activated protein kinase (MAPK) pathway. To identify new regulators of the filamentous growth MAPK pathway, a genetic screen was performed with a collection of 4072 nonessential deletion mutants constructed in the filamentous (Σ1278b) strain background. The screen, in combination with directed gene-deletion analysis, uncovered 97 new regulators of the filamentous growth MAPK pathway comprising 40% of the major regulators of filamentous growth. Functional classification extended known connections to the pathway and identified new connections. One function for the extensive regulatory network was to adjust the activity of the filamentous growth MAPK pathway to the activity of other pathways that regulate the response. In support of this idea, an unregulated filamentous growth MAPK pathway led to an uncoordinated response. Many of the pathways that regulate filamentous growth also regulated each other’s targets, which brings to light an integrated signaling network that regulates the differentiation response. The regulatory network characterized here provides a template for understanding MAPK-dependent differentiation that may extend to other systems, including fungal pathogens and metazoans.
Keywords: signal transduction, signal integration, response coordination, network hubs, signal coordination
Cell differentiation is the process by which cells undergo specialization to produce different cell types with different functions. Cell-type specialization can result from execution of an intrinsic developmental program and also in response to extrinsic cues. The process of cell differentiation is one of exquisite precision: cells undergo complete morphogenetic restructuring in a specific spatiotemporal context (Kholodenko et al. 2010). Multiple signaling pathways collaborate to control cell differentiation responses. For example, the activity of the Wnt and Hippo pathways is integrated at multiple levels to coordinate development (McNeill and Woodgett 2010). A critical problem in the field of cell differentiation is to elucidate how signals from different pathways become integrated to produce a cohesive response. This problem is relevant from the standpoint of human health, because misregulation of differentiation pathways is an underlying cause of developmental problems and diseases such as cancer (Wagner and Nebreda 2009).
Depending on ploidy and growth condition, the budding yeast Saccharomyces cerevisiae can differentiate into different cell types. Haploid yeast undergoes morphological changes in response to secreted pheromones to mate and form diploids (Bardwell 2005; Dohlman and Slessareva 2006; Merlini et al. 2013). Diploid yeast starved for carbon and nitrogen initiate a meiotic program known as sporulation (Neiman 2011). Haploid and diploid yeast starved for only carbon or nitrogen undergoes filamentous (or invasive/pseudohyphal) growth (Gimeno et al. 1992; Cullen and Sprague 2000, 2012). During filamentous growth, major changes occur to cell polarity (Gimeno et al. 1992; Roberts and Fink 1994; Pruyne and Bretscher 2000; Cullen and Sprague 2002; Bi and Park 2012), cell-cycle progression (Kron et al. 1994; Edgington et al. 1999), and cell adhesion (Lambrechts et al. 1996; Lo and Dranginis 1998; Guo et al. 2000), which results in formation of branched chains of interconnected invasive filaments. Filamentous cells form complex communities during biofilm formation (Reynolds and Fink 2001; Verstrepen and Klis 2006; Bojsen et al. 2012). Many fungal species undergo filamentous growth. In pathogens, differentiation to filamentous/hyphal cells in biofilms is critical for pathogenicity (Lo et al. 1997; Wendland 2001; Nobile et al. 2006; Sohn et al. 2006). Budding yeast therefore provides a convenient genetic system to define the pathways that regulate filamentous growth and has provided insights into the genetic basis of fungal pathogenesis and eukaryotic differentiation.
Signal transduction pathways regulate filamentous growth and control the changes that occur in response to nutrient limitation (Zhao et al. 2007). Among the pathways that regulate filamentous growth in yeast is a MAPK pathway called the filamentous growth MAPK pathway (Supporting Information, Figure 1A). MAPK pathways are evolutionary conserved signaling modules that regulate diverse responses in eukaryotes (Raman et al. 2007). The filamentous growth MAPK pathway is composed of plasma-membrane sensors (Msb2p, Sho1p, and Opy2p) (O’Rourke and Herskowitz 1998; Cullen et al. 2004; Wu et al. 2006; Yamamoto et al. 2010; Karunanithi and Cullen 2012) that connect to a Rho-type GTPase (Cdc42p; Bi and Park 2012) and a kinase cascade consisting of a p21-activated kinase (Ste20p; Peter et al. 1996; Leberer et al. 1997) and MAPK module (including the MAPKKK Ste11p, MAPKK Ste7p, and MAPK Kss1p; Roberts and Fink 1994). The MAP kinase Kss1p regulates the activity of two transcription factors (Ste12p and Tec1p; Madhani and Fink 1997; Madhani et al. 1997) that induce target genes (Madhani et al. 1999) by binding to well-defined promoter elements (Zeitlinger et al. 2003; Chou et al. 2006).
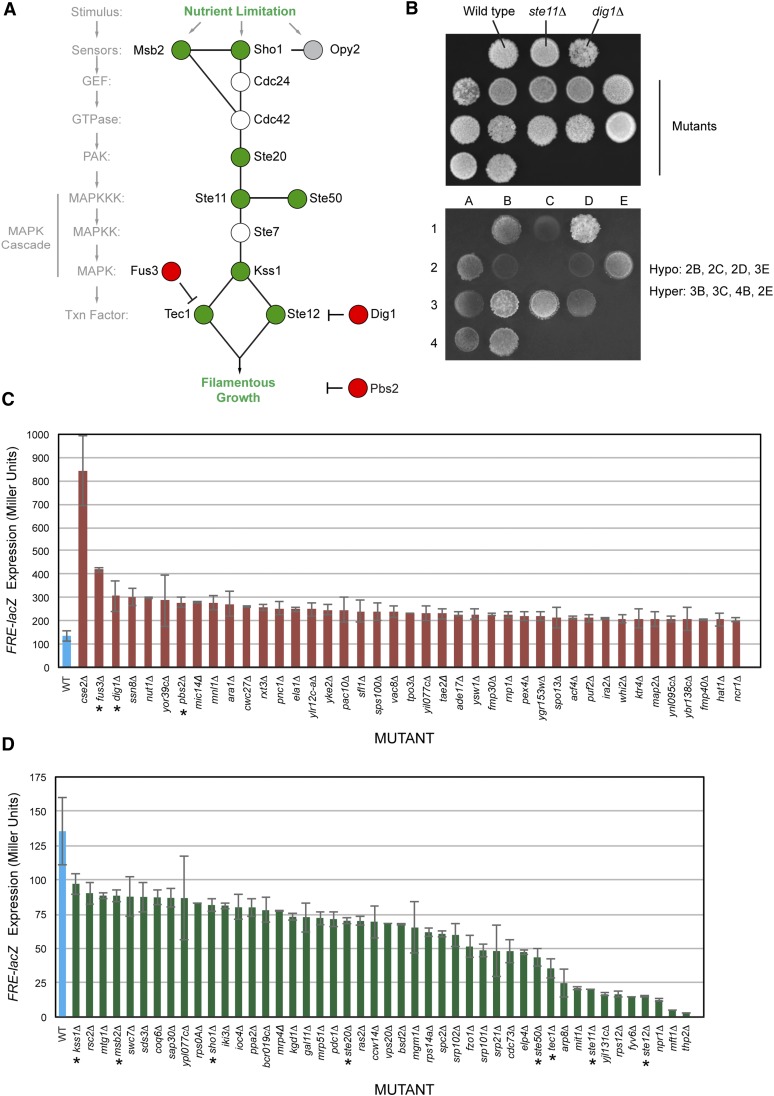
Figure 1.
Positive and negative regulators of the filamentous growth MAPK pathway. (A) The filamentous growth MAPK pathway is shown with components that were identified by the screen represented as positive regulators (green circles) and negative regulators (red circles). Cdc24p and Cdc42p are essential proteins and were not tested here, and Ste7p was not present in the collection. Opy2p is an established regulator of the filamentous growth MAPK pathway (Yang et al. 2009, 2010; Karunanithi and Cullen 2012) and showed a defect in FRE–lacZ expression, but the levels fell below the range of statistical significance (Table S3). (B) Example of the plate-washing assay. Equal concentrations of cells were spotted onto YEPD media. Cells were grown for 2 days. Plates were photographed (top) and washed in a stream of water to reveal invaded cells (bottom). Examples of hyper- and hypoinvasive growth mutants are listed at right in reference to strains lacking a positive (ste11Δ) and negative (dig1Δ) regulator. (C) Mutants showing elevated pFRE–lacZ expression and hyperinvasive growth. β-Galactosidase assays were performed in at least duplicate. Blue bar, wild-type control strain; red bars, mutants tested. Values are expressed in Miller units (U). Error bars represent standard deviation between independent trials. P-values and raw data provided in Table S3. (D) Mutants showing reduced pFRE–lacZ expression and reduced invasive growth. Blue bar, wild-type control strain; green bars, mutants tested. See C for details. The pFRE–lacZ activity of the kss1Δ did not fall within the 1.5-fold cutoff but is shown as a reference.
In addition to the MAPK pathway, other pathways also regulate filamentous growth. Major nutrient regulatory pathways include the Ras2p–cAMP–protein kinase A (PKA) pathway (Toda et al. 1985; Gimeno et al. 1992; Mosch et al. 1996; Colombo et al. 1998; Robertson and Fink 1998; Mosch et al. 1999; Rupp et al. 1999), the AMP-dependent kinase (AMPK) Snf1p and transcriptional repressors Nrg1p and Nrg2p (Celenza and Carlson 1989; Woods et al. 1994; Lesage et al. 1996; Cullen and Sprague 2000; McCartney and Schmidt 2001; Kuchin et al. 2002), the target of rapamycin (TOR) pathway, which responds to nitrogen availability (Beck and Hall 1999; Cardenas et al. 1999; Bruckner et al. 2011), and the mitochondrial retrograde (RTG) pathway (Sekito et al. 2002; Liu et al. 2003; Liu and Butow 2006), which senses changes in metabolic respiration (Aun et al. 2013). The pH sensing Rim101p pathway (Lamb et al. 2001; Barrales et al. 2008), lipid-responsive transcription factor Opi1p (White et al. 1991; Reynolds 2006), tRNA modification complex Elongator [ELP, (Krogan and Greenblatt 2001; Winkler et al. 2001; Petrakis et al. 2004; Li et al. 2007; Svejstrup 2007)], and chromatin remodeling complex Rpd3p(L) (Carrozza et al. 2005; Barrales et al. 2008; Ryan et al. 2012) also regulate filamentous growth. These proteins represent only a subset of a large collection of regulators identified in S. cerevisiae and Candida albicans by gene expression profiling (Madhani et al. 1999; Roberts et al. 2000; Carlisle and Kadosh 2013), genetic screens (Lorenz et al. 2000; Palecek et al. 2000; Barrales et al. 2008), and systematic genome-wide approaches, including large-scale deletion and overexpression studies (Jin et al. 2008; Bharucha et al. 2011; Shively et al. 2013), mass spectrometry (MASS SPEC) approaches (Xu et al. 2010; Zhang et al. 2013) and analysis of ordered deletion collections made in the filamentous (Σ1278b) background (Dowell et al. 2010; Ryan et al. 2012). A critical challenge is to understand how many different proteins and pathways come together to produce a new cell type.
Several of the pathways that regulate filamentous growth can regulate each other’s activities. A landmark finding comes from the discovery that the Ras2p pathway regulates the filamentous growth MAPK pathway (Mosch et al. 1996). More recently, the ELP (Abdullah and Cullen 2009), Rim101, RTG, Rpd3p(L), and Opi1p pathways have also been shown to regulate the filamentous growth MAPK pathway (Chavel et al. 2010). These pathways control expression of the gene encoding one of the plasma-membrane sensors for the filamentous growth MAPK pathway, Msb2p (Chavel et al. 2010). It has also been shown that the major transcription factors that regulate filamentous growth regulate each other's targets, which creates hubs where signal integration events are coordinated (Borneman et al. 2006). One hub is the FLO11 promoter, where multiple transcription factors converge to fine tune cell adhesion (Rupp et al. 1999). Likewise, the major protein kinases that regulate filamentous growth function in an interdependent network (Bharucha et al. 2008). Therefore, signal coordination occurs at multiple levels to regulate the filamentous growth response.
Here we examine the question of signal integration by performing a genetic screen with an ordered deletion collection in the filamentous (Σ1278b) background (Ryan et al. 2012). This effort, combined with hypothesis-based testing, identified 97 new regulators of the filamentous growth MAPK pathway, which map to known regulatory pathways and provide entirely new connections. Using the screen as a platform, we examin questions related to network connectivity. We show that tuning the activity of the filamentous growth MAPK pathway to the other pathways is critical to producing a coordinated response. We also show that several of the key pathways that regulate filamentous growth also regulate each other's targets. Thus, an integrated network regulates the filamentous growth response. We speculate that similarly coordinated networks coordinate cell differentiation responses in other systems.
Materials and Methods
Strains, plasmids, and microbiological techniques
Filamentous growth was evaluated in the Σ1278b strain background (Liu et al. 1996). The haploid gene deletion collection constructed in the Σ1278b strain background has been described (Ryan et al. 2012) and was generously provided by C. Boone. pFRE–lacZ was provided by H. Madhani (Madhani et al. 1997). The YCp–Cdc12–GFP was provided by J. Pringle (Fares et al. 1996). The pste12::URA3 plasmid was provided from G. Sprague (McCaffrey et al. 1987). p8XCRE–lacZ was provided by H. Saito (Tatebayashi et al. 2006). pCIT2–lacZ was provided by the Liu lab and has been described (Liu and Butow 1999).
Standard laboratory conditions were used to grow yeast and bacterial cultures (Rose et al. 1990). Escherichia coli was grown in LB and 2XYT media. Yeast was grown in rich media YEPD (2% glucose) or YEP–GAL (2% galactose) or synthetic complete media at 30° unless otherwise noted. Yeast strains are listed in Table 1. Gene deletions were constructed using antibiotic resistance markers (Goldstein and McCusker 1999) or auxotrophic markers amplified by PCR and introduced into yeast by lithium acetate transformation by standard methods as described (Chavel et al. 2010). The plate washing (Roberts and Fink 1994) and single-cell invasive growth assays (Cullen and Sprague 2000) were used to measure filamentous growth. Colony morphology was examined by visual inspection on YEPD media (Granek and Magwene 2010; Voordeckers et al. 2012). Functional analysis of the MAPK regulatory genes came from SGD (http://www.yeastgenome.org).
Table 1. Yeast strains used in this study.
| Strain | Genotype | Reference |
|---|---|---|
| PC313a | MATa ura3-52 | Liu et al. (1993) |
| PC538 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 | Cullen et al. (2004) |
| PC539 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 ste12::KLURA3 | Cullen et al. (2004) |
| PC563 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 bud8::KLURA3 | Cullen and Sprague (2002) |
| PC586 | MATα ura3-52 leu2 | Cullen et al. (2004) |
| PC622 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 GAL-SHO1 | Cullen et al. (2004) |
| PC949 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pop2::KanMX6 | This study |
| PC950 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 ccr4::KanMX6 | This study |
| PC999 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA | Cullen et al. (2004) |
| PC1083 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA GAL-MSB2::KanMX6 | Cullen et al. (2004) |
| PC1415 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 bni1::KLURA3 | Cullen and Sprague (2002) |
| PC1516 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HAΔ100-818 | Cullen et al. (2004) |
| PC1558 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 sho1::HYG ssk1::NAT | Pitoniak et al. (2009) |
| PC1621 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HAΔ100-818 GAL-SHO1::GENT | This study |
| PC1625 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 GAL-MSB2-HA::NAT GAL-SHO1::GENT | This study |
| PC1895 | MATa ura3-52 leu2::HYG | This study |
| PC2043 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 FLO11-HA::KanMX6 | Karunanithi et al. (2010) |
| PC2061 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 ssk1::NAT ste11::KLURA3 | Pitoniak et al. (2009) |
| PC2112 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 leu2::HYG lacZ::NAT tec1::LEU2 | Vadaie et al. (2008) |
| PC2360 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 ras2::NAT | Chavel et al. (2010) |
| PC2362 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 ira1::NAT | Chavel et al. (2010) |
| PC2511 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 ras2::NAT ste12::KLURA3 | This study |
| PC2515 | MATα ura3-52 leu2 flo8::NAT | Chavel et al. (2010) |
| PC2532 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 flo8::HYG | Chavel et al. (2010) |
| PC2534 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pde2::HYG | Chavel et al. (2010) |
| PC2535 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 gpa2::NAT | Chavel et al. (2010) |
| PC2537b | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 gpr1::KLURA3 | Chavel et al. (2010) |
| PC2588 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 tpk1::NAT | Chavel et al. (2010) |
| PC2618 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 grr1::KLURA3 | This study |
| PC2622 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 snf8::HYG | This study |
| PC2633 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 sdc25::NAT | Chavel et al. (2010) |
| PC2688 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA ste12::KLURA3 | This study |
| PC2690 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA ras2::KLURA3 | This study |
| PC2763 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 elp2::KLURA3 | Abdullah and Cullen (2009) |
| PC2845 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 FLO11-HA::KanMX6 gal11:KLURA3 | This study |
| PC2945 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA rxt2::KLURA3 | Chavel et al. (2010) |
| PC2952 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA hda1::KLURA3 | Chavel et al. (2010) |
| PC2953 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA rim101::KLURA3 | Chavel et al. (2010) |
| PC2954 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA snf2::KLURA3 | Chavel et al. (2010) |
| PC2955 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA msn1::KLURA3 | Chavel et al. (2010) |
| PC2956 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA yak1::KLURA3 | Chavel et al. (2010) |
| PC2957 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA mss11::KLURA3 | Chavel et al. (2010) |
| PC2980 | MATa ura3-52 elp2::KLURA3 | This study |
| PC3016 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 bem4::HYG | A. Pitoniak, C. Chavel, J. Chow, j. Smith, D. Camara, S. Karunanithi, K. Wolfe, K., and P. J. Cullen, (unpublished data) |
| PC3030 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA sin3::NAT | Chavel et al. (2010) |
| PC3031 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA isw1::NAT | Chavel et al. (2010) |
| PC3032 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA cka1::NAT | Chavel et al. (2010) |
| PC3033 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA nhp10::NAT | Chavel et al. (2010) |
| PC3034 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA isw2::NAT | Chavel et al. (2010) |
| PC3035 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA mks1::NAT | Chavel et al. (2010) |
| PC3037 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA sds3::KLURA3 | Chavel et al. (2010) |
| PC3038 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA rpd3::KLURA3 | Chavel et al. (2010) |
| PC3039 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA dig1::KLURA3 | Chavel et al. (2010) |
| PC3352 | MATa ura3-52 ras2::NAT | This study |
| PC3353 | MATa ura3-52 sin3::NAT | This study |
| PC3362 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA fkh1::KLURA3 | Chavel et al. (2010) |
| PC3363 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA nrg1::KLURA3 | Chavel et al. (2010) |
| PC3414 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 FLO11-HA spo14::KLURA3 | Karunanithi et al. (2010) |
| PC3415 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 FLO11-HA dfg16::KLURA3 | Karunanithi et al. (2010) |
| PC3419 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 FLO11-HA ash1::KLURA3 | Karunanithi et al. (2010) |
| PC3421 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 FLO11-HA plb3::KLURA3 | Karunanithi et al. (2010) |
| PC3428 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA swi4::KLURA3 | Chavel et al. (2010) |
| PC3429 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA mga1::KLURA3 | Chavel et al. (2010) |
| PC3430 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA fkh2::NAT | Chavel et al. (2010) |
| PC3431 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA sfl1::KLURA3 | Chavel et al. (2010) |
| PC3432 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA fkh1::KLURA3 fkh2::NAT | Chavel et al. (2010) |
| PC3435 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA nrg1::KLURA3 nrg2::NAT | Chavel et al. (2010) |
| PC3635 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 bud3::KLURA3 | This study |
| PC3637 | MATα ura3-52 leu2 ste12::kanMX6 | This study |
| PC3642 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA rtg3::NAT | Chavel et al. (2010) |
| PC3643 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA tco89::NAT | Chavel et al. (2010) |
| PC3644 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA gzf3::NAT | Chavel et al. (2010) |
| PC3652 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA rtg2::NAT | Chavel et al. (2010) |
| PC3654 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA tor1::NAT | Chavel et al. (2010) |
| PC3657 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA lsc2::NAT | This study |
| PC3687 | MATα ura3-52 leu2 opi1::NAT | This study |
| PC3688 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA opi1::NAT | Chavel et al. (2010) |
| PC3690 | MATα ura3-52 leu2 rim101::NAT | This study |
| PC3691 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA rim101::NAT | This study |
| PC3695 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA rtg1::NAT | This study |
| PC3861 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 ste11::NAT | Karunanithi and Cullen (2012) |
| PC3920 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA vps8::NAT | This study |
| PC4006 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 mdh1::KLURA3 | This study |
| PC4007 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 spt3::KLURA3 | This study |
| PC4008 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 spt8::KLURA3 | This study |
| PC4032 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 rim20::KLURA3 | This study |
| PC4035 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 bmh2::KLURA3 | This study |
| PC4038 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 cmk2::KLURA3 | This study |
| PC4039 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 cmk1::HYG | This study |
| PC4043 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 bmh1::KLURA3 | This study |
| PC4141 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 tpk2::KLURA3 | This study |
| PC4256 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 bud1::NAT | This study |
| PC4468 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 rga1::KLURA3 | This study |
| PC5071 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pcl9::NAT | This study |
| PC5072 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pcl1::KLURA3 plc9::NAT | This study |
| PC5073 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pcl2::HYG plc9::NAT | This study |
| PC5074 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pcl1::KLURA3 pcl2::HYG pcl9::NAT | This study |
| PC5075 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pcl1::KLURA3 plc2::HYG | This study |
| PC5084 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA tpk3::NAT | This study |
| PC5085 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 gln3::KLURA3 | This study |
| PC5090 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 nte1::NAT | This study |
| PC5091 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pho85::NAT | This study |
| PC5095 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA sir2::NAT | This study |
| PC5102 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA gcn5::KLURA3 | This study |
| PC5108 | MATa ura3-52 tpk2::NAT | This study |
| PC5111 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 stb3::NAT | This study |
| PC5113 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 stb6::NAT | This study |
| PC5115 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pho4::NAT | This study |
| PC5121 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pho80::NAT | This study |
| PC5332 | MATa ura3-52 rtg2::NAT | This study |
| PC5335 | MATa ura3-52 pho85::KLURA3 | This study |
| PC5340 | MATα ura3-52 leu2 gcn5::LEU2 | This study |
| PC5351 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA ygr125w::NAT | This study |
| PC5352 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA gpb2::KLURA3 | This study |
| PC5354 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA cup2::KLURA3 | This study |
| PC5360 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA aca1::KLURA3 | This study |
| PC5362 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA sko1::KLURA3 | This study |
| PC5364 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA plb1::TRP | This study |
| PC5651 | MATa ura3-52 ste12::NAT | This study |
| PC5822 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 vps27::KLURA3 | This study |
| PC5826 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 vps26::KLURA3 | This study |
| PC5831 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 vps35::KKLURA3 | This study |
| PC5856 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 hap4::KLURA3 | This study |
| PC5860 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 mep2::KLURA3 | This study |
| PC5862 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 rim15::KLURA3 | This study |
| PC5865 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 sch9::KLURA3 | This study |
| PC5871 | MATa ura3-52 leu2::HYG ssk1::NAT | This study |
| PC5872 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 crz1::KLURA3 | This study |
| PC5875 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pho81::KLURA3 | This study |
| PC5876 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pho84::KLURA3 | This study |
| PC5878 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 rim21::KLURA3 | This study |
| PC5880 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 rim9::KLURA3 | This study |
| PC5881 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pho5:KLURA3 | This study |
| PC6016c | MATa can1Δ::Ste2pr-spHIS5 lyp1Δ::Ste3pr-LEU2 his3::hisG leu2Δ0 ura3Δ0 | Ryan et al. (2012) |
| PC6093 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 gpb1::NAT gpb2::KLURA3 | This study |
| PC6103 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA tec1::NAT | This study |
| PC6135 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 rsr1::HIS3 bni1::KLURA3 | This study |
| PC6136 | MATa ura3-52 pde2::NAT | This study |
| PC6137 | MATa ura3-52 ccr4::NAT | This study |
| PC6138 | MATa ura3-52 nte1::NAT | This study |
| PC6139 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 ino1::KLURA3 | This study |
| PC6140 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 smp1::KLURA3 | This study |
| PC6141 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 flo1::KLURA3 | This study |
| PC6159 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 ccr4::NAT ssk1::KLURA3 | This study |
| PC6161 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 nte1::NAT ssk1::KLURA3 | This study |
| PC6163 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pde2::NAT ssk1::KLURA3 | This study |
| PC6165 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 pho85::NAT ssk1::KLURA3 | This study |
| PC6166 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 ras2::NAT ssk1::KLURA3 | This study |
| PC6192 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 faa4::KLURA3 | This study |
| PC6193 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA hac1::KLURA3 | This study |
| PC6197 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA stb5::NAT | This study |
| PC6198 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA stp1::KLURA3 | This study |
| PC6201 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA skn7::KLURA3 | This study |
| PC6202 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA yhp1::KLURA3 | This study |
| PC6204 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA mot3::NAT | This study |
| PC6206 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA stb2::NAT | This study |
| PC6208 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA ace2::KLURA3 | This study |
| PC6210 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA azf1::NAT | This study |
| PC6212 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA phd1::NAT | This study |
| PC6218 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA sok2::KLURA3 | This study |
| PC6222 | MATa ura3-52 ras2::NAT | This study |
| PC6253 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 bcy1::KLURA3 | This study |
| PC6258 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 MSB2-HA mot2::KLURA3 | This study |
| PC6284 | MATa can1Δ::Ste2pr-spHIS5 lyp1Δ::Ste3pr-LEU2 his3::hisG leu2Δ0 ura3Δ0 mcy1::kanMX4 ste12::LEU2 | This study |
| PC6285 | MATa can1Δ::Ste2pr-spHIS5 lyp1Δ::Ste3pr-LEU2 his3::hisG leu2Δ0 ura3Δ0 pnc1::kanMX4 ste12::LEU2 | This study |
| PC6286 | MATa can1Δ::Ste2pr-spHIS5 lyp1Δ::Ste3pr-LEU2 his3::hisG leu2Δ0 ura3Δ0 mnl1::kanMX4 ste12::LEU2 | This study |
| PC6287 | MATa can1Δ::Ste2pr-spHIS5 lyp1Δ::Ste3pr-LEU2 his3::hisG leu2Δ0 ura3Δ0 rxt3::kanMX4 ste12::LEU2 | This study |
| PC6288 | MATa can1Δ::Ste2pr-spHIS5 lyp1Δ::Ste3pr-LEU2 his3::hisG leu2Δ0 ura3Δ0 cwc27::kanMX4 ste12::LEU2 | This study |
| PC6289 | MATa can1Δ::Ste2pr-spHIS5 lyp1Δ::Ste3pr-LEU2 his3::hisG leu2Δ0 ura3Δ0 ssn8::kanMX4 ste12::LEU2 | This study |
| PC6290 | MATa can1Δ::Ste2pr-spHIS5 lyp1Δ::Ste3pr-LEU2 his3::hisG leu2Δ0 ura3Δ0 nut1::kanMX4 ste12::LEU2 | This study |
| PC6291 | MATa can1Δ::Ste2pr-spHIS5 lyp1Δ::Ste3pr-LEU2 his3::hisG leu2Δ0 ura3Δ0 ela1::kanMX4 ste12::LEU2 | This study |
| PC6292 | MATα ura3-52 leu2 flo8::NAT ssk1::KLURA3 | This study |
| PC6293 | MATα ura3-52 leu2 gcn5::LEU2 ssk1::KLURA3 | This study |
| PC6294 | MATα ura3-52 leu2 opi1::NAT ssk1::KLURA3 | This study |
| PC6295 | MATα ura3-52 leu2 rim101::NAT ssk1::KLURA3 | This study |
| PC6296 | MATa ura3-52 sin3::NAT ssk1::KLURA3 | This study |
| PC6297 | MATa ste4 FUS1-lacZ FUS1-HIS3 ura3-52 leu2::HYG lacZ::NAT tec1::LEU2 | This study |
| PC6298 | MATα ura3-52 leu2 elp2::LEU2 | This study |
| PC6299 | MATa ura3-52 leu2::HYG ssk1::NAT elp2::LEU2 | This study |
All strains are in the Σ1278b background unless otherwise indicated.
KLURA3 refers to the Kluyveromyces lactis URA3 cassette.
Σ1278b ordered deletion collection control strain MATa can1Δ::Ste2pr-spHIS5 lyp1Δ::Ste3pr-LEU2 his3::hisG leu2Δ0 ura3Δ.
Halo assays were performed as described (Jenness et al. 1987). Specifically, wild-type and mutant strains were grown to saturation in YEPD (2% glucose). Cell density was determined by OD A600. Cultures were serially diluted such that ∼10,000 cells were spread onto YEPD plates. After the cell suspension had dried, four spots of 1 µg/µl alpha factor were spotted (10 µl). Plates were incubated at 30° for 2 days and photographed.
Invasive growth screen of the filamentous deletion collection
The MATa Σ1278b deletion collection (Ryan et al. 2012) was pinned in 96-well format to YEPD media omnitrays (Thermo Scientific, Waltham, MA). Each plate was pinned independently using a pinning tool (V&P Scientific, VP408, San Diego, CA)—sterilized with a 10% bleach solution, 95% ethanol, 70% ethanol and flame—and a pinning guide tray (V&P Scientific, VP381). Plates were pinned in duplicate and incubated for 5 and 12 days. Plates were photographed, washed in a stream of water, and photographed again. Each plate was scored visually for colonies that showed changes in morphology and invasive growth. Scores were tabulated to produce a single score called the invasive growth index. The results of the screen and details of the scoring system are presented in Table S2.
Evaluating filamentous growth MAPK pathway activity
The activity of the filamentous growth MAPK pathway was evaluated with a transcriptional reporter [pFRE–lacZ (Madhani and Fink 1997)]. Strains that showed clear-cut invasive growth phenotypes (hyper- and hypoinvasive) were transformed with the pFRE–lacZ plasmid. Strains were grown in media lacking uracil to maintain selection for the plasmid and the nonpreferred carbon source galactose (S-GAL–URA) to induce pathway activity (Pitoniak et al. 2009). Mutants were induced in S-GAL–URA for 4 hr. Mutants were grown in batches of ∼20 alongside control strains (tec1Δ and dig1Δ) to minimize batch-to-batch variation. Cell extracts were prepared, and β-galactosidase assays were performed as described (Chavel et al. 2010). The average values of at least two independent experiments were reported. Statistical significance was determined by comparing the difference between wild-type and experimental pFRE–lacZ expression averages in a z-test score (Freedman et al. 1998). The z-test score was converted to the P-value (http://www.graphpad.com). Samples with a P-value ≤0.0001 and ≥1.5-fold change from wild type were considered statistically significant. Raw data for the β-galactosidase assays can be found in Table S3. For some experiments, the filamentous growth MAPK pathway was evaluated with a growth reporter [FUS1–HIS3, (McCaffrey et al. 1987)]. In Σ1278b cells lacking an intact mating pathway (ste4Δ), growth on SD–HIS is dependent on the filamentous growth MAPK pathway (O’Rourke and Herskowitz 1998; Cullen et al. 2004; Pitoniak et al. 2009; Chavel et al. 2010; Karunanithi and Cullen 2012). Growth assays are shown in Figure S1. As a separate test, 26 genes identified by the screen were disrupted in a wild-type Σ1278b strain and checked for invasive growth and pFRE–lacZ; 77% passed a preliminary test.
Budding pattern analysis
Patterns of bud-site selection were based on established principles (Chant and Pringle 1995). Budding pattern was determined in two ways. In one method, budding pattern was based on visual inspection of connected cells. Σ1278b cells grown in liquid YEPlowD (0.2% glucose) media undergo filamentous growth and exhibit Ste12p-dependent changes in cell length, cell–cell adhesion, and distal-unipolar budding. Cells were grown to midlog phase in YEPlowD (0.2% glucose) liquid medium for 12–14 hr and examined by microscopy at 100× magnification. Buds were assigned as proximal, equatorial, or distal depending on their position relative to mother cells. At least 150 cells were counted for each experiment.
In a separate approach, cells were stained by FITC-ConA and TRITC-ConA based on published protocols (Matheos et al. 2004; Gao and Bretscher 2009) with the following modifications. Cells were grown in YEPlowD for 16 hr. FITC-ConA (0.1 mg/ml) was added to 1 ml cells. Cells were incubated in the dark for 15 min, washed three times, and resuspended in YEPlowD for 4 hr. Cells (1 ml) were then stained with TRITC-ConA (0.1 mg/ml), washed three times in water, and examined by fluorescence microscopy to visualize the position of buds. At least 200 buds were recorded for each condition.
Quantitative PCR analysis
Quantitative PCR (qPCR) analysis was performed as described (Pfaffl 2001). To prepare total RNA, cells were grown in 50-ml aliquots in YEP–GAL medium to midlog phase (∼6 hr). Total RNA was isolated by hot acid phenol extraction. cDNA synthesis and real-time PCR reactions were performed as described (Chavel et al. 2010). qPCR and melt curve data collection was performed as described (Chavel et al. 2010) with the following alterations to the amplification cycles: initial denaturation for 3 min at 95°, followed by 35× cycle 3 (denaturation for 30 sec at 95°, annealing for 30 sec at 60°, and extension for 30 sec at 72°). Gene expression was quantified using the ΔΔCt method as described (Livak and Schmittgen 2001). All reactions were performed in triplicate, and average values are reported. Primers used are as follows: ACT1 (forward 5′-GGCTTCTTTGACTACCTTCCAACA-3′ and reverse 5′-GATGGACCACTTTCGTCGTATTC-3′), NRG1 (forward 5′-CTAATGATGCATATAATAAGATGGC-3′ and reverse 5′-ATGACCCGATGTAGTGAATCCT-3′), PHO5 (forward 5′-ACATCACCTTGCAGACTGTCA-3′ and reverse 5′-AAGTACTAGCGTCAGTTGAGG-3′), INO1 (forward 5′-CTAATCAAGATGAGAGAGCCAAT-3′ and reverse 5′-ATACTTCTACGTACCTCTCAGTA-3′), and SMP1 (forward 5′-AGTCAAGATTCCTCCAGTGTAC-3′ and reverse 5′-ATCCGCTCGTGATATTGCTC-3′).
Fluorescence microscopy
Actin staining by rhodamine phalloidin has been described (Amberg 2000). Differential interference contrast (DIC) and fluorescence microscopy using rhodamine and GFP filter sets were performed using an Axioplan 2 fluorescent microscope (Zeiss, Jena, Germany) with a PLAN-APOCHROMAT 100X/1.4 (oil) objective (N.A. 0.17). Digital images were obtained with the Axiocam MRm camera (Zeiss). Axiovision 4.4 software (Zeiss) was used for image acquisition and analysis.
Results
Identification of filamentous growth MAPK pathway regulators
An ordered collection of 4072 deletion mutants constructed in the filamentous (MATa Σ1278b) background (provided by the Boone Lab, Toronto, ON; Ryan et al. 2012) was screened for changes in colony morphology (Granek and Magwene 2010; Voordeckers et al. 2012) and invasive growth based on the plate-washing assay (Roberts and Fink 1994) to identify regulators of filamentous growth. These assays provide a readout of filamentous growth that correlate with the activity of the filamentous growth MAPK pathway (Figure 1B; Roberts and Fink 1994).
Screens were performed at two time periods (5 and 12 days), which allowed evaluation of the progression of invasive growth. The 5-day screen was better suited to identify hyperfilamentous growth mutants, and the 12-day screen enriched for hypofilamentous growth mutants. The two screens also provided independent validation of the mutants identified (Table S2). A scoring system incorporated colony morphology and agar invasion from both screens into a single value called the invasive growth index that was used to rank the mutants by strength-of-phenotype (Table S2).
Of the 4072 mutants represented in the collection, 220 showed hyperfilamentous growth and 478 showed hypofilamentous growth (Table S2). Many of these mutants have been identified in other screens (Table S1) (Lambrechts et al. 1996; Lo and Dranginis 1998; Pan and Heitman 1999; Lorenz et al. 2000; Ryan et al. 2012; Shively et al. 2013). The screen uniquely identified new regulators of filamentous growth (Table S1), which may have been due to the specific incubation times or differences in scoring systems.
To identify those regulators of filamentous growth that also regulate the filamentous growth MAPK pathway, mutants identified in the screen were examined for changes in the activity of a transcriptional reporter, pFRE–lacZ, which provides a readout of filamentous growth MAPK pathway activity (Madhani and Fink 1997; Pitoniak et al. 2009). Control strains verified that loss of negative regulators showed elevated pFRE–lacZ activity (Figure 1, A and C, asterisks, and Table S3), which included the transcriptional repressor Dig1p (Cook et al. 1996), the mating pathway MAP kinase Fus3p (Bruckner et al. 2004), and the HOG pathway MAP kinase kinase Pbs2p (Figure 1, A and C and Table S3; asterisks in panel C refers to pathway components). Loss of pathway components showed reduced pFRE–lacZ activity (Figure 1, A and D, asterisks; msb2Δ, sho1Δ, ste50Δ, tec1Δ, ste20Δ, ste11Δ, kss1Δ, and ste12Δ; Table S3).
Mutants identified by the invasive growth screens were transformed with pFRE–lacZ reporter and evaluated for β-galactosidase activity. For the hyperinvasive growth mutants, 41 of 110 showed elevated pFRE–lacZ expression (37%, Figure 1C). For the hypoinvasive growth mutants, 43 of 116 tested showed a defect in pFRE–lacZ expression (37%, Figure 1D). Not all candidates were examined, because mutants with weaker phenotypes showed differences that fell below the statistical cutoff employed (≥1.5-fold, P-value ≤ 0.0001). Thus, the screen was not saturating.
To validate the results of the screen, and/or extend connections of known pathways to the filamentous growth MAPK pathway, ∼100 genes were disrupted in a wild-type Σ1278b strain, and gene disruptants were evaluated for invasive growth and MAPK activity (Table S1 and Figure S1). The analysis was facilitated by a cross-talk reporter that in a mating-deficient strain (ste4Δ FUS1–HIS3) provides a readout of the filamentous growth MAPK pathway. The analysis eliminated ∼15% of the candidates as false positives (Table S1). The analysis also identified several new components. In total, 97 proteins were identified by the screen and gene disruption analysis that regulate the filamentous growth MAPK pathway and play a corresponding role in the regulation of filamentous growth.
Evaluating filamentous growth MAPK pathway regulators by the change in budding pattern and cell elongation
Candidate regulators were examined for morphological phenotypes that are controlled by the filamentous growth MAPK pathway. The filamentous growth MAPK pathway regulates changes in budding pattern (Gimeno et al. 1992; Roberts and Fink 1994; Cullen and Sprague 2000, 2002) and the cell cycle that results in an increase in cell length (Kron et al. 1994; Madhani et al. 1999). The change in budding pattern is visually striking in haploid cells that switch from axial to distal-unipolar budding (Cullen and Sprague 2002). A recent study showed an abundance of filamentous cells in MAPK pathway mutants, raising the question of whether and to what extent the MAPK pathway regulates this aspect of the response (Chen and Thorner 2010). To clarify this issue, the budding pattern of filamentous cells was examined by two approaches. In one approach, filamentous growth was examined in liquid culture by microscopy (Figure 2A, left columns). In a second approach, cells were stained with FITC-ConA and TRITC-ConA at different times to visualize bud position (Figure 2A, right columns). The latter approach had the advantage of determining bud position without the assumption of which cell the parent was (Figure 2B). The approaches were in close agreement for wild-type cells and control strains lacking axial [bud3Δ (Chant et al. 1991)], distal [bud8Δ (Harkins et al. 2001; Schenkman et al. 2002; Kang et al. 2004), and core [rsr1Δ (Park et al. 1997, 2002; Kang et al. 2010)] bud-site-selection markers (Figure 2A).
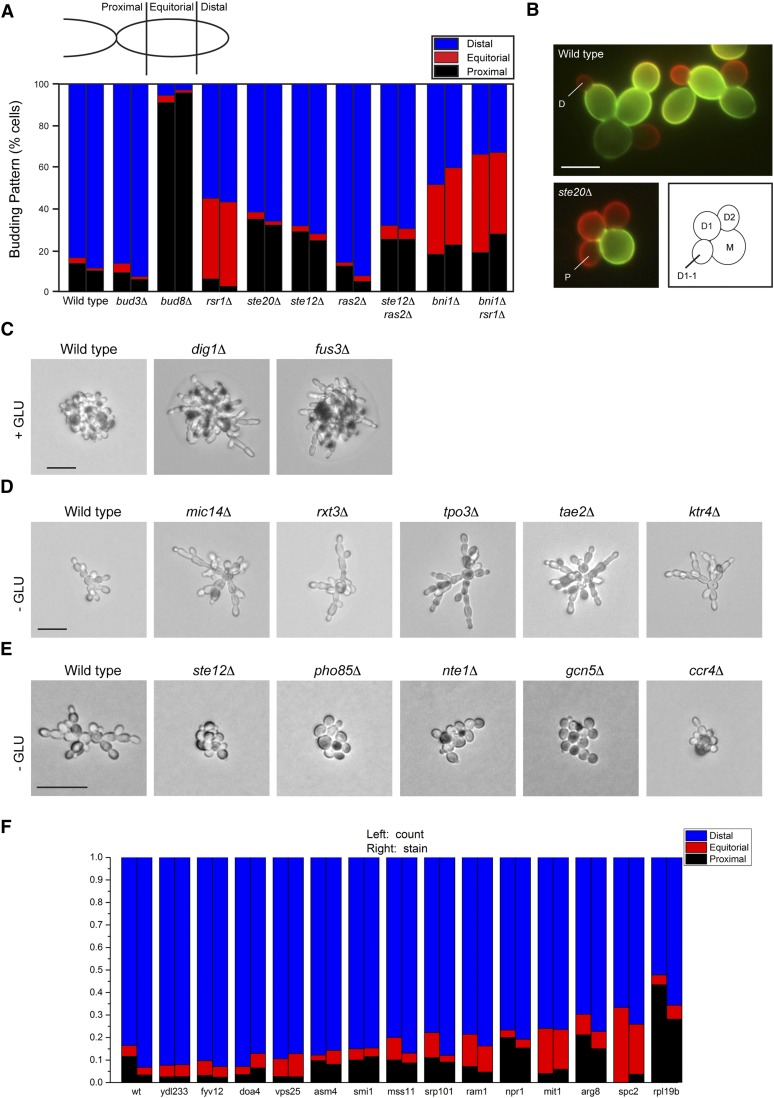
Figure 2.
Role of filamentous growth MAPK pathway regulators in bud-site selection and filament formation. (A) Bar graph, the percentage of cells exhibiting distal (blue), equatorial (red), or proximal (black) budding pattern. Left column: assignment based on visual inspection. Right column: assignment based on fluorescence microscopy. (B) Example of cells costained with FITC/TRITC-ConA. Top: wild-type cells. Bottom left: the ste20Δ mutant. Bar, 5 μm. Bottom right: cartoon representing budding cells. D, distal; P, proximal. (C) Single-cell assay shows hyperpolarized growth and distal-pole budding pattern of the fus3Δ and dig1Δ mutants in medium containing 2% glucose (HIGH GLU). Bar, 20 μm. (D) Single-cell assay of select hyperinvasive growth mutants. Cells were grown on S-GLU (-GLU) medium at low density for 16 hr and photographed at 100×. Bar, 20 μm. Representative microcolonies are shown; other examples are in Figure S2. (E) Single-cell assay of select hypoinvasive growth mutants. Bar, 20 μm. Other mutants are shown in Figure S2. (F) Budding pattern analysis of hypofilamentous growth mutants indicated. Scoring system is the same as in A.
Wild-type haploid cells showed a characteristic change in budding pattern from axial to distal-unipolar budding when grown in glucose-limited medium (Figure 2A) (Chant and Pringle 1995; Cullen and Sprague 2002)]. The ste12Δ mutant showed a 15% reduction in distal-unipolar budding, and the ste20Δ mutant showed a 20% reduction (Figure 2A). Many cells retained distal-pole budding (60%), which can account for the conclusion that the filamentous growth MAPK pathway is dispensable (Chen and Thorner 2010). Therefore, the filamentous growth MAPK pathway regulates the change in polarity during filamentous growth. Other signaling pathways probably also regulate the change in budding pattern. Under this condition, the ras2Δ mutant did not play a role (Figure 2A). The rsr1Δ mutant did not show a completely random budding pattern, but retained the propensity to bud at the distal pole. This may be due to increased polarized growth of filamentous cells, which bias bud-site-selection to the distal pole (Sheu et al. 2000; Cullen and Sprague 2002). Disruption of the gene encoding the formin Bni1p, which reduces the polarized growth of filamentous cells (Cullen and Sprague 2002), conferred random budding to the rsr1Δ mutant (Figure 2B, bni1Δ rsr1Δ).
The single-cell invasive-growth assay provides a convenient measure of the changes in budding pattern and cell length that occur during filamentous growth (Cullen and Sprague 2000) and was used to examine mutants identified in the screen. Most hyperinvasive growth mutants showed hyperelongated morphology by the single-cell assay. Glucose suppresses the filamentous morphology (Cullen and Sprague 2000) and effectively suppressed hyperelongated morphology and distal-pole budding pattern in all but two hyperinvasive growth mutants, dig1Δ and fus3Δ (Figure 2C). These mutants regulate the filamentous growth MAPK pathway; thus, a hyperactive filamentous growth MAPK pathway can bypass the inhibition of cell elongation and distal-pole budding induced by growth of cells in high-glucose environments.
Other hyperinvasive growth mutants showed hyperelongated cell morphology (Figure 2D and Figure S2). A subset of these were dependent on Ste12p for invasive growth and morphology (Figure S3, A and B). Not all mutants showed Ste12p dependence (Figure S3), which might reflect a role for these proteins in regulating filamentous growth outside the MAPK pathway. Hypoinvasive growth mutants were similarly examined. Most hypoinvasive growth mutants, like the ste12Δ mutant, showed a defect in unipolar budding and cell elongation (Figure 2E and Figure S2). Similarly, a subset of mutants showed defects in distal-pole budding (Figure 2F). Whereas no mutant was completely defective, several (like the ste12Δ mutant) showed minor differences. It is possible that distal-pole budding during filamentous growth results from the additive contribution of multiple pathways. Therefore, the budding pattern and single-cell analysis corroborated a role for many of these proteins in regulating the filamentous growth MAPK pathway.
Functional analysis of candidate genes and pathways
The genes identified by the screen and directed gene deletion analysis were classified by GO annotation terms for biological process, cellular compartment, and molecular function (Ashburner et al. 2000). Genes were also overlaid onto known protein and genetic interaction maps (Uetz et al. 2000; Drees et al. 2001; Ho et al. 2002; Miller et al. 2005; Costanzo et al. 2010). As a result, the genes were found to comprise functional categories that were explored in detail below (Figure 3).
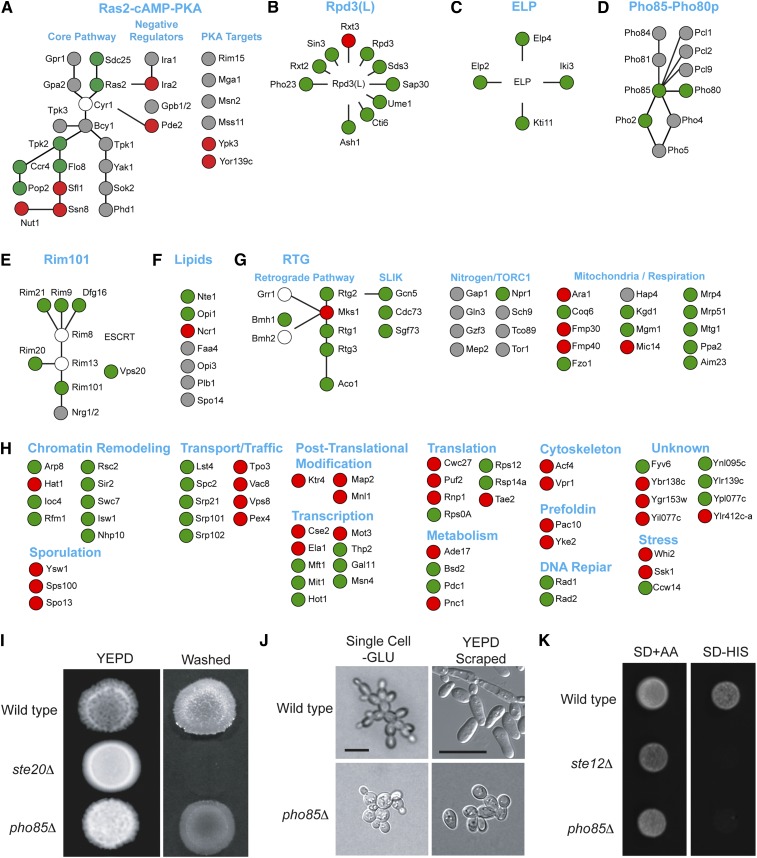
Figure 3.
Functional classification of filamentous growth MAPK pathway regulators. Genes identified by the screen (Figure 1, C and D) or by hypothesis-based testing (Figure S1) are shown according to their established roles in pathways or protein complexes. Lines refer to functional connections. Green, positive regulator; red, negative regulator; gray, no phenotype; and white, not tested. (A) The Ras2p–cAMP–PKA pathway; (B) Rpd3p(L) chromatin remodeling complex (Table S3); (C) the ELP complex; (D) the Pho85p–Pho80p pathway; (E) the Rim101 pathway. (F) Lipid biosynthesis; (G) the RTG pathway and proteins associated with mitochondrial function; and (H) Functional classification of other proteins. (I) Plate-washing assay for pho85Δ mutant alongside controls. (J) Single-cell assay for the pho85Δ mutant alongside controls. Scraped refers to cells scraped from an invasive scar. (K) FUS1–HIS3 reporter activity for the pho85Δ mutant alongside controls.
A subset of Ras2p pathway regulators was found to regulate the filamentous growth MAPK pathway. These include Ras2p (Mosch et al. 1996), the alternative GEF Sdc25p (Damak et al. 1991; Jones et al. 1991), the phosphodiesterase Pde2p, and PKA subunit Tpk2p (Figure 3A; Chavel et al. 2010). Tpk2p regulates the transcription factor Flo8p (Robertson and Fink 1998) and negatively regulates the transcriptional repressor Sfl1p (Conlan and Tzamarias 2001; Pan and Heitman 2002). Flo8p and Sfl1p were identified by the screen (Figure 3A). Sfl1p-interacting proteins Ssn8p and Nut1p are required for Sfl1p to carry out its role as a transcriptional repressor (Conlan and Tzamarias 2001) and were identified by the screen (Figure 3A). Ccr4p, a component of the Ccr4p–NOT deadenylase complex, which is an effector of Tpk2p (Lenssen et al. 2002) and target of the MAP kinase Kss1p (Fasolo et al. 2011) was also identified.
The screen uncovered components of the chromatin remodeling complex Rpd3p(L) (Figure 3B, Ash1p, Sap30p, Ume1p, Cti6p, Pho23p, and Rxt3p; Rpd3p, Sin3p, Rxt2p, and Sds3p were previously identified; Chavel et al. 2010). The ELP complex regulates the filamentous growth MAPK pathway (Figure 3C; Abdullah and Cullen 2009; Elp2p, Elp6p, Iki3p, Kti12p). The screen identified members of the ELP complex (Figure 3C, Elp3p, Iki3p, and Elp4p). Thus, the screen was effective at identifying regulatory connections to the filamentous growth MAPK pathway and supports the idea that the Ras2p pathway, Rpd3p(L), and ELP are major regulators of the filamentous growth MAPK pathway.
The screen identified the cyclin-dependent kinase Pho85p (Figure 3, D and I–K and Figure S1) (Measday et al. 1997; Huang et al. 2002, 2007; Shemer et al. 2002; Moffat and Andrews 2004). Loss of cyclins Pcl1p, Pcl2p, and Pcl9p, and the triple pcl1Δ pcl2Δ pcl9Δ mutant, showed no defect in filamentous growth MAPK pathway activity (Figure 3D and Figure S1), whereas loss of Pho80p, the cyclin responsible for environmental responses controlled by Pho85p (Liu et al. 2000), showed reduced filamentous growth MAPK pathway activity (Figure 3D). Deletion of the transcription factor PHO2 (Kaffman et al. 1994; O’Neill et al. 1996; Liu et al. 2000) showed reduced filamentous growth MAPK pathway activity (Figure 3D).
Several proteins and pathways were not identified by the screen but were shown to regulate the filamentous growth MAPK pathway by direct testing. These may have been missed by the screen for several reasons. One is that they were not represented in the collection. This was true for components of Rpd3p(L), including tod6Δ and ume6Δ. A second reason is that the pathways may play a conditional role in regulating the filamentous growth MAPK pathway. For example, the Ras2p pathway does not constitutively regulate the filamentous growth MAPK pathway (Figure S4), which complicated assessment of the roles of Flo8p and Tpk2p. A third reason is that phenotypes may have fallen below threshold of statistical significance applied to the data (e.g., tpk2Δ, rim101Δ, kss1Δ, opy2Δ, rim9Δ, dfg16Δ, rim13Δ, rim8Δ, ume1Δ; Table S3).
Components of the Rim101p pathway were found to regulate the filamentous growth MAPK pathway (Figure 3E, Chavel et al. 2010). Nrg1p and Nrg2p (Lamb and Mitchell 2003) did not regulate the filamentous growth MAPK pathway (Figure 3E). The lipid regulatory transcription factor Opi1p (Greenberg et al. 1982) regulates the filamentous growth MAPK pathway (Chavel et al. 2010). The serine esterase Nte1p, which serves as the phospholipase B of yeast (Fernandez-Murray et al. 2009) was also found to regulate the filamentous growth MAPK pathway (Figure 3F). Ncr1p, which regulates sphingolipid biosynthesis (Malathi et al. 2004) and has not been shown to interact with Opi1p or Nte1p genetically or physically, negatively regulated the filamentous growth MAPK pathway.
The RTG pathway regulates the filamentous growth MAPK pathway (Figure 3G, Chavel et al. 2010). The screen did not identify this pathway but did uncover proteins that influence mitochondrial function (Figure 3G). A main target of RTG is aconitase (Liu and Butow 1999), an enzymatic component of the TCA cycle necessary to generate α-ketoglutarate, a precursor in glutamate biosynthesis (Magasanik and Kaiser 2002). Aco1p positively regulated the filamentous growth MAPK pathway (Figure 3G). Rtg2p is also incorporated into the histone-acetyl transferase (HAT) complex SLIK, with the HAT Gcn5p as its catalytic component (Pray-Grant et al. 2002). Deletion of GCN5 reduced filamentous growth MAPK pathway activity (Figure 3G). Npr1p, a kinase that stabilizes amino acid transporters at the membrane (De Craene et al. 2001) and is negatively regulated by the TORC1 complex (Schmidt et al. 1998), was found to positively regulate the filamentous growth MAPK pathway.
The above analysis accounted for nearly half the proteins identified by the screen and involved the Ras2p, Rpd3p(L), ELP, Opi1p, Rim101p, RTG, and Pho85p pathways. The remaining proteins represent new connections to the filamentous growth MAPK pathway. These included proteins that regulate transcription [including components of the THO complex and chromatin remodeling proteins that are separate from Rpd3p(L)], protein transport and trafficking (including components of the signal recognition complex, SRP), protein translation, prefoldin, metabolism, sporulation, the cytoskeleton, post-translational modification, and genes whose functions remain to be characterized (Figure 3H).
Although it is not clear how these pathways regulate the filamentous growth MAPK pathway, the majority of regulators tested did not influence the activity of the mating pathway (Figure S5) or the HOG pathway (Figure S6A), although a HOG pathway reporter p8XCRE–lacZ was modestly induced in some hyperinvasive growth mutants (Figure S6B). These pathways share components with the filamentous growth MAPK pathway (Chen and Thorner 2007; Saito 2010; Saito and Posas 2012). Thus, it would appear that these factors by and large play a specific role in regulating the filamentous growth MAPK pathway. We previously showed that many pathways converge on the expression of the MSB2 promoter (Chavel et al. 2010). Perhaps these regulators regulate the filamentous growth MAPK pathway in a similar manner.
Unregulated filamentous growth MAPK pathway activity is detrimental to invasive growth and proper morphogenesis
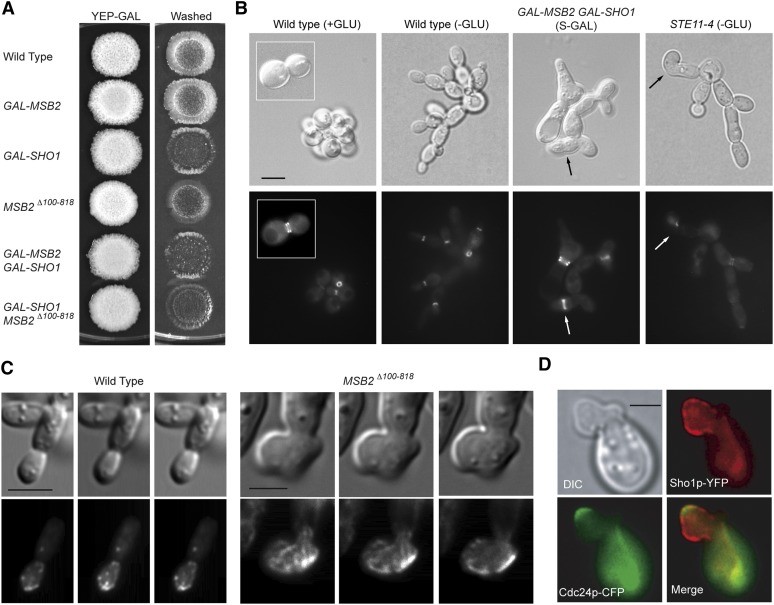
It is not entirely clear why the regulation of the filamentous growth MAPK pathway is so extensive. One possibility is that the activity of the filamentous growth MAPK pathway may be adjusted to that of other pathways that regulate filamentous growth. Coordination of morphogenetic pathways that regulate cell-cycle progression and cell polarity might be critical, for example, for proper growth. To test this possibility, the activity of the filamentous growth MAPK pathway was genetically separated from other regulatory pathways using gain-of-function alleles and by driving expression of pathway regulators with inducible promoters. Hyperactive alleles of MSB2 (Cullen et al. 2004), SHO1 (Vadaie et al. 2008), and STE11 (Stevenson et al. 1992) were examined. In addition, overexpression of pathway components was assessed with the strong inducible promoter (pGAL; Longtine et al. 1998). Preliminary observations with these strains showed that hyperactivation or overexpression of Sho1p or Msb2p did not induce hyperinvasive growth but rather caused a reduction in invasive growth (Figure 4A). Microscopic examination showed that the cells had morphological defects (Figure 4, B–D).
Figure 4.
Overexpression/hyperactivation of pathway components does not enhance filamentous growth. (A) Plate-washing assay was performed for the indicated strains on YEP–GAL. GAL–MSB2 and GAL–SHO1 strains where the GAL1 promoter has replaced the native promoter. MSB2Δ100–818 is a hyperactive allele of MSB2. (B) Septin staining. Strains containing the pCdc12p–GFP plasmid were grown on SCD or S medium lacking glucose. Bar, 5 μm. (C) Indicated strains were stained with rhodamine phalloidin. Bar, 5 μm. (D) Colocalization of Sho1p and Cdc24p in cells carrying a hyperactive allele of MSB2. Bar, 5 μm.
To further explore this question, cell-polarity markers for the mother-bud neck (with the septin Cdc12p–GFP) and the cytoskeleton (with rhodamine phalloidin, which stains actin) were examined in these mutants. Septin staining showed defects in cytokinesis (Figure 4B). Actin staining showed irregular patterns, with polymerized actin at multiple surface sites on the plasma membrane (Figure 4C, File S1, and File S2). Moreover, the localization of polarity control proteins, Sho1p and Cdc24p, were localized to aberrant structures in these mutants (Figure 4D). The prevalence of these phenotypes varied among the mutants tested and ranged from ∼10% irregular morphologies for the STE11-4 mutant to >90% irregular cells in the GAL–MSB2 GAL–SHO1 mutant. Prolonged overexpression of SHO1 resulted in growth defects (not shown). Equivalent morphologies were observed in other mutants that exhibited filamentous growth MAPK pathway hyperactivation (not shown). However, these phenotypes stood out from most of the hyperfilamentous growth mutants identified in the screen, possibly because pGAL-driven and hyperactive proteins have higher pathway activity. Therefore, hyperactivation of the filamentous growth MAPK pathway causes problems with normal cell morphogenesis. A likely explanation for this phenotype is that it results from that pathway’s critical roles in cell-polarity and cell-cycle control. Therefore, coordination of the activity of the filamentous growth MAPK pathway may be necessary not only to promote a coherent filamentation response but also to maintain proper cell growth.
Pathways that regulate filamentous growth control each other’s targets
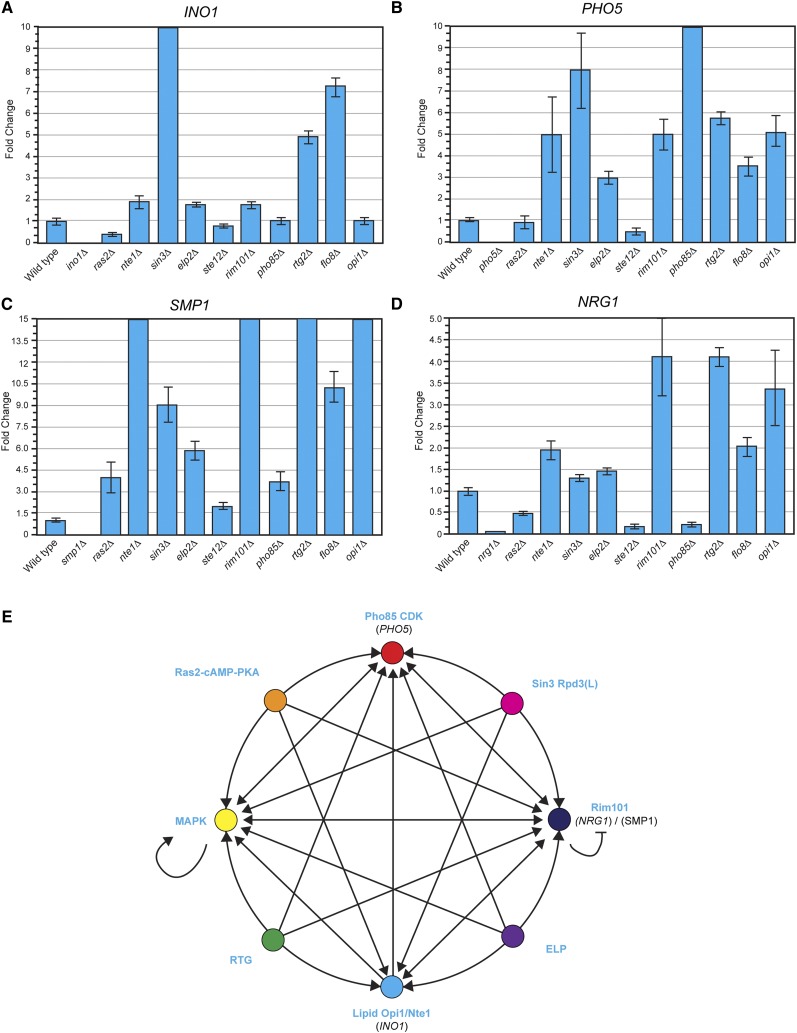
The filamentous growth MAPK pathway may be the terminal pathway at which many pathways converge. An alternative possibility that has not been explored is that many of the pathways that regulate filamentous growth may also regulate each other’s activities. To test this possibility, transcriptional targets of several of the major pathways that regulate filamentous growth were evaluated in a panel of pathway mutants by qPCR analysis and/or transcriptional reporters. NRG1 and SMP1 were selected as targets of the Rim101p pathway, which are downregulated by that pathway and upregulated in that mutant (Lamb and Mitchell 2003); PHO5, which is downregulated by the Pho85p pathway and upregulated in the mutant (Kaffman et al. 1994); CIT2, which is a target of the RTG pathway (Rothermel et al. 1995); and INO1, which is downregulated by the lipid/Opi1p pathway (White et al. 1991). qPCR and/or lacZ analysis was performed in mutants lacking the major filamentation control pathways (ras2Δ, nte1Δ, sin3Δ, elp2Δ, ste12Δ, rim101Δ, pho85Δ, rtg2Δ, flo8Δ, and opi1Δ). With the exception of Opi1p/INO1, each pathway regulated the expression of its own target (Figure 5, A–D). Many pathways similarly influenced the expression of each other’ targets. For example, INO1’s expression was upregulated in the nte1Δ, sin3Δ, elp2Δ, rim101Δ, rtg2Δ, and flo8Δ mutants (Figure 5A). Thus, in some manner, the Nte1p, Sin3p, Epl2p, Rim101p, Rtg2p, and Flo8p proteins contribute to the coregulation of a lipid pathway target. Similar results were found for PHO5 (Figure 5B), SMP1 (Figure 5C), and NRG1 (Figure 5D). Several pathways were also found to regulate the RTG pathway, including Ras2p and ELP, based on CIT2–lacZ reporter (Figure S7). DNA microarray analysis previously identified a major transcriptional target of the filamentous growth MAPK pathway as the gene encoding Rim8p, a component of the Rim101p pathway (Chavel et al. 2010), and we confirmed that the filamentous growth MAPK pathway contributed to RIM8 expression by qPCR analysis (data not shown). These results indicate that a subset of the major pathways that regulate filamentous growth regulate at least one of each other’s key targets.
Figure 5.
Signal integration between pathways during filamentous growth. (A–D) Quantitative PCR analysis of labeled genes in the indicated mutants. The bar indicates change in expression using the ΔΔCt quantitation method. Wild-type expression levels were set to 1. The error bars represent standard deviation between experiments. Cells were grown to midlog phase in YEP–GAL media for 6 hr. (E) Diagram showing connections between pathways that regulate filamentous growth. Arrows refer to positive regulation. Bars, negative regulation.
The mechanisms by which such regulation occurs is not clear and may occur through diverse means such as pathway-to-pathway connections or the modulation of transcription factors that serve as master regulators of signaling outputs. Moreover, not all of the possible regulatory connections were observed. For example, the filamentous growth MAPK pathway does not appear to regulate Ras2p–cAMP–PKA (Chavel et al. 2010) or the RTG pathways (Figure S7). Nevertheless, the results are striking from the perspective that each arrow represents in principle a regulatory connection that occurs between two pathways (Figure 5E).
Discussion
Cell differentiation involves the combined action of many different proteins and pathways. How multiple signals become integrated into a cohesive response is an important biological problem that in many cases remains unclear. Here, we explore the question of signal integration by identifying, from a global perspective, regulators of the MAPK pathway responsible for controlling differentiation to the filamentous cell type. Using a genetic screen and direct testing, we identify >95 proteins that when absent influence the activity of the filamentous growth MAPK pathway. This number likely represents an underestimate because the screen was not saturating and because a rigorous statistical cutoff was used to establish regulators. In addition, the screen was performed under a single condition where some pathways and complexes may not be required. A conservative estimate is that >35% of the major regulators of filamentous growth regulate the activity of the filamentous growth MAPK pathway.
One consequence of these regulatory connections is to sensitize MAPK activity to different stimuli. Many of the major nutrient-regulatory pathways in yeast, such as TOR (Bruckner et al. 2011), Snf1p (Karunanithi and Cullen 2012), Ras2p (this study; Mosch et al. 1996; Chavel et al. 2010), and RTG (this study; Chavel et al. 2010) impinge on the activity of the filamentous growth MAPK pathway. We also show that pathways that sense and respond to diverse stimuli, such as pH (Rim101p) and other environmental stimuli (Pho85p) also regulate the filamentous growth MAPK pathway. The connection between Pho85p and the filamentous growth MAPK pathway is particularly relevant as Pho85p has been shown in C. albicans to be required for temperature-dependent filamentation (Shapiro et al. 2012).
A second reason that the filamentous growth MAPK pathway is extensively regulated might be to coordinate its activity with other pathways that regulate the same response. In this way, the MAPK-dependent changes to budding pattern, the cell cycle, and cell adhesion can be tuned to the global network. Hence, multiple pathways can tap into these major regulatory events (instead of each pathway making changes directly). The filamentous growth MAPK pathway regulates the change in budding that occurs during filamentous growth. Mutants that reduce pathway activity (ste20 and ste12) show a decrease in distal-pole budding, and mutants with elevated pathway activity (dig1 and fus3) show an increase in distal-pole budding, even under high-glucose conditions. Given that multiple pathways regulate distal-pole budding, it is likely that the role of the filamentous growth MAPK pathway may be as significant as the contributions of other pathways. We show that unregulated filamentous growth MAPK pathway activity is detrimental to proper morphogenesis and cell growth.
We also show that extensive cross-regulation occurs among several of the pathways that regulate filamentous growth. Our study highlights a degree of signal integration that has not been previously appreciated. The qPCR performed here (Figure 5) was under conditions in which the filamentous growth MAPK pathway is activated (e.g., poor carbon sources, like galactose). The network described in Figure 5A was examined under a single growth condition. It is possible that other connections between pathways were missed if they occur under a condition that was not tested. Genetic buffering between the pathways may obscure connections. Similarly, loss of one pathway may or may not induce loss of the filamentous growth phenotype. The ways by which this regulation is accomplished is not clear. Multiple pathways could feed into a central metabolite or small molecule (cAMP) that regulates a master regulatory transcription factor, from which multiple filamentation targets are controlled. Indeed, transcriptional “hub” proteins globally regulate filamentous growth (Borneman et al. 2006). At least one connection may be direct. RIM8 is a major target of the filamentous growth MAPK pathway (Chavel et al. 2010). As not all pathways regulate each other’s targets, the connections between the pathways are presumably specific.
In conclusion, filamentous growth results from a highly coordinated and integrated signaling network. A single MAPK pathway regulates filamentous growth, which is controlled by many different pathways to integrate various signals and coordinate the response. Signal integration is commonly seen in similar differentiation responses in higher eukaryotes, where multiple stimuli activate an interconnected set of signaling pathways (Cuenda and Rousseau 2007; Katz et al. 2007; Raman et al. 2007). Perhaps the connections identified here extend to related pathways in other systems.
Supplementary Material
Acknowledgments
Thanks go to C. Boone (University of Toronto), H. Madhani (UCSF), G. Sprague (University of Oregon), Z. Liu (University of New Orleans), H. Saito (University of Tokyo), and J. Pringle (Stanford University) for providing strains, strain collections, plasmids, and/or suggestions. The work was supported by a grant from the National Institutes of Health (GM098629).
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.168252/-/DC1.
Communicating editor: C. Boone
Literature Cited
- Abdullah U., Cullen P. J., 2009. The tRNA modification complex elongator regulates the Cdc42-dependent mitogen-activated protein kinase pathway that controls filamentous growth in yeast. Eukaryot. Cell 8: 1362–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg, D. C., D. J. Burke, and J. N. Strathern, 2000 Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Press: 1–230. [Google Scholar]
- Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., et al. , 2000. Gene ontology: tool for the unification of biology. Nat. Genet. 25: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aun A., Tamm T., Sedman J., 2013. Dysfunctional mitochondria modulate cAMP-PKA signaling and filamentous and invasive growth of Saccharomyces cerevisiae. Genetics 193: 467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell L., 2005. A walk-through of the yeast mating pheromone response pathway. Peptides 26: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrales R. R., Jimenez J., Ibeas J. I., 2008. Identification of novel activation mechanisms for FLO11 regulation in Saccharomyces cerevisiae. Genetics 178: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck T., Hall M. N., 1999. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402: 689–692. [DOI] [PubMed] [Google Scholar]
- Bharucha N., Ma J., Dobry C. J., Lawson S. K., Yang Z., et al. , 2008. Analysis of the yeast kinome reveals a network of regulated protein localization during filamentous growth. Mol. Biol. Cell 19: 2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha N., Chabrier-Rosello Y., Xu T., Johnson C., Sobczynski S., et al. , 2011. A large-scale complex haploinsufficiency-based genetic interaction screen in Candida albicans: analysis of the RAM network during morphogenesis. PLoS Genet. 7: e1002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E., Park H. O., 2012. Cell polarization and cytokinesis in budding yeast. Genetics 191: 347–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojsen R. K., Andersen K. S., Regenberg B., 2012. Saccharomyces cerevisiae: a model to uncover molecular mechanisms for yeast biofilm biology. FEMS Immunol. Med. Microbiol. 65: 169–182. [DOI] [PubMed] [Google Scholar]
- Borneman A. R., Leigh-Bell J. A., Yu H., Bertone P., Gerstein M., et al. , 2006. Target hub proteins serve as master regulators of development in yeast. Genes Dev. 20: 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner S., Kohler T., Braus G. H., Heise B., Bolte M., et al. , 2004. Differential regulation of Tec1 by Fus3 and Kss1 confers signaling specificity in yeast development. Curr. Genet. 46: 331–342. [DOI] [PubMed] [Google Scholar]
- Bruckner S., Kern S., Birke R., Saugar I., Ulrich H. D., et al. , 2011. The TEA transcription factor Tec1 links TOR and MAPK pathways to coordinate yeast development. Genetics 189: 479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas M. E., Cutler N. S., Lorenz M. C., Di Como C. J., Heitman J., 1999. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 13: 3271–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle P. L., Kadosh D., 2013. A genome-wide transcriptional analysis of morphology determination in Candida albicans. Mol. Biol. Cell 24: 246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrozza M. J., Florens L., Swanson S. K., Shia W. J., Anderson S., et al. , 2005. Stable incorporation of sequence specific repressors Ash1 and Ume6 into the Rpd3L complex. Biochim. Biophys. Acta 1731: 77–87. [DOI] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M., 1989. Mutational analysis of the Saccharomyces cerevisiae SNF1 protein kinase and evidence for functional interaction with the SNF4 protein. Mol. Cell. Biol. 9: 5034–5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J., Pringle J. R., 1995. Patterns of bud-site selection in the yeast Saccharomyces cerevisiae. J. Cell Biol. 129: 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J., Corrado K., Pringle J. R., Herskowitz I., 1991. Yeast BUD5, encoding a putative GDP-GTP exchange factor, is necessary for bud site selection and interacts with bud formation genes BEM1. Cell 65: 1213–1224. [DOI] [PubMed] [Google Scholar]
- Chavel C. A., Dionne H. M., Birkaya B., Joshi J., Cullen P. J., 2010. Multiple signals converge on a differentiation MAPK pathway. PLoS Genet. 6: e1000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. E., Thorner J., 2007. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1773: 1311–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. E., Thorner J., 2010. Systematic epistasis analysis of the contributions of protein kinase A- and mitogen-activated protein kinase-dependent signaling to nutrient limitation-evoked responses in the yeast Saccharomyces cerevisiae. Genetics 185: 855–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S., Lane S., Liu H., 2006. Regulation of mating and filamentation genes by two distinct Ste12 complexes in Saccharomyces cerevisiae. Mol. Cell. Biol. 26: 4794–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo S., Ma P., Cauwenberg L., Winderickx J., Crauwels M., et al. , 1998. Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J. 17: 3326–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan R. S., Tzamarias D., 2001. Sfl1 functions via the co-repressor Ssn6-Tup1 and the cAMP-dependent protein kinase Tpk2. J. Mol. Biol. 309: 1007–1015. [DOI] [PubMed] [Google Scholar]
- Cook J. G., Bardwell L., Kron S. J., Thorner J., 1996. Two novel targets of the MAP kinase Kss1 are negative regulators of invasive growth in the yeast Saccharomyces cerevisiae. Genes Dev. 10: 2831–2848. [DOI] [PubMed] [Google Scholar]
- Costanzo M., Baryshnikova A., Bellay J., Kim Y., Spear E. D., et al. , 2010. The genetic landscape of a cell. Science 327: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A., Rousseau S., 2007. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 1773: 1358–1375. [DOI] [PubMed] [Google Scholar]
- Cullen P. J., Sprague G. F., Jr, 2000. Glucose depletion causes haploid invasive growth in yeast. Proc. Natl. Acad. Sci. USA 97: 13619–13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P. J., Sprague G. F., Jr, 2002. The roles of bud-site-selection proteins during haploid invasive growth in yeast. Mol. Biol. Cell 13: 2990–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P. J., Sprague G. F., Jr, 2012. The regulation of filamentous growth in yeast. Genetics 190: 23–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P. J., Sabbagh W., Jr, Graham E., Irick M. M., van Olden E. K., et al. , 2004. A signaling mucin at the head of the Cdc42- and MAPK-dependent filamentous growth pathway in yeast. Genes Dev. 18: 1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak F., Boy-Marcotte E., Le-Roscouet D., Guilbaud R., Jacquet M., 1991. SDC25, a CDC25-like gene which contains a RAS-activating domain and is a dispensable gene of Saccharomyces cerevisiae. Mol. Cell. Biol. 11: 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Craene J. O., Soetens O., Andre B., 2001. The Npr1 kinase controls biosynthetic and endocytic sorting of the yeast Gap1 permease. J. Biol. Chem. 276: 43939–43948. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Slessareva J. E., 2006. Pheromone signaling pathways in yeast. Sci. STKE 2006: cm6. [DOI] [PubMed] [Google Scholar]
- Dowell R. D., Ryan O., Jansen A., Cheung D., Agarwala S., et al. , 2010. Genotype to phenotype: a complex problem. Science 328: 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees B. L., Sundin B., Brazeau E., Caviston J. P., Chen G. C., et al. , 2001. A protein interaction map for cell polarity development. J. Cell Biol. 154: 549–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington N., Blacketer M., Bierwagen T., Myers A., 1999. Control of Saccharomyces cervisiae filamentous growth by cyclin-dependent kinase Cdc28. Mol. Cell. Biol. 19: 1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares H., Goetsch L., Pringle J. R., 1996. Identification of a developmentally regulated septin and involvement of the septins in spore formation in Saccharomyces cerevisiae. J. Cell Biol. 132: 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasolo J., Sboner A., Sun M. G., Yu H., Chen R., et al. , 2011. Diverse protein kinase interactions identified by protein microarrays reveal novel connections between cellular processes. Genes Dev. 25: 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Murray J. P., Gaspard G. J., Jesch S. A., McMaster C. R., 2009. NTE1-encoded phosphatidylcholine phospholipase b regulates transcription of phospholipid biosynthetic genes. J. Biol. Chem. 284: 36034–36046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D., Piscani D., Durves R., 1998. Statistics. Norton, New York. [Google Scholar]
- Gao L., Bretscher A., 2009. Polarized growth in budding yeast in the absence of a localized formin. Mol. Biol. Cell 20: 2540–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno C. J., Ljungdahl P. O., Styles C. A., Fink G. R., 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68: 1077–1090. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., McCusker J. H., 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15: 1541–1553. [DOI] [PubMed] [Google Scholar]
- Granek J. A., Magwene P. M., 2010. Environmental and genetics determinants of colony morphology in yeast. PLoS Genet. 6: e1000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. L., Reiner B., Henry S. A., 1982. Regulatory mutations of inositol biosynthesis in yeast: isolation of inositol-excreting mutants. Genetics 100: 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Styles C. A., Feng Q., Fink G. R., 2000. A Saccharomyces gene family involved in invasive growth, cell-cell adhesion, and mating. Proc. Natl. Acad. Sci. USA 97: 12158–12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins H. A., Page N., Schenkman L. R., De Virgilio C., Shaw S., et al. , 2001. Bud8p and Bud9p, proteins that may mark the sites of bipolar bud-site selection in yeast. Mol. Biol. Cell 12: 2497–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y., Gruhler A., Heilbut A., Bader G. D., Moore L., et al. , 2002. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415: 180–183. [DOI] [PubMed] [Google Scholar]
- Huang D., Moffat J., Andrews B., 2002. Dissection of a complex phenotype by functional genomics reveals roles for the yeast cyclin-dependent protein kinase Pho85 in stress adaptation and cell integrity. Mol. Cell. Biol. 22: 5076–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Friesen H., Andrews B., 2007. Pho85, a multifunctional cyclin-dependent protein kinase in budding yeast. Mol. Microbiol. 66: 303–314. [DOI] [PubMed] [Google Scholar]
- Jenness D. D., Goldman B. S., Hartwell L. H., 1987. Saccharomyces cerevisiae mutants unresponsive to alpha-factor pheromone: alpha-factor binding and extragenic suppression. Mol. Cell. Biol. 7: 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R., Dobry C. J., McCown P. J., Kumar A., 2008. Large-scale analysis of yeast filamentous growth by systematic gene disruption and overexpression. Mol. Biol. Cell 19: 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Vignais M. L., Broach J. R., 1991. The CDC25 protein of Saccharomyces cerevisiae promotes exchange of guanine nucleotides bound to ras. Mol. Cell. Biol. 11: 2641–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffman A., Herskowitz I., Tjian R., O’Shea E. K., 1994. Phosphorylation of the transcription factor PHO4 by a cyclin-CDK complex, PHO80–PHO85. Science 263: 1153–1156. [DOI] [PubMed] [Google Scholar]
- Kang P. J., Angerman E., Nakashima K., Pringle J. R., Park H. O., 2004. Interactions among Rax1p, Rax2p, Bud8p, and Bud9p in marking cortical sites for bipolar bud-site selection in yeast. Mol. Biol. Cell 15: 5145–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P. J., Beven L., Hariharan S., Park H. O., 2010. The Rsr1/Bud1 GTPase interacts with itself and the Cdc42 GTPase during bud-site selection and polarity establishment in budding yeast. Mol. Biol. Cell 21: 3007–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanithi S., Cullen P. J., 2012. The filamentous growth MAPK pathway responds to glucose starvation through the Mig1/2 transcriptional repressors in Saccharomyces cerevisiae. Genetics 192: 869–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanithi S., Vadaie N., Chavel C. A., Birkaya B., Joshi J., et al. , 2010. Shedding of the mucin-like flocculin Flo11p reveals a new aspect of fungal adhesion regulation. Curr. Biol. 20: 1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M., Amit I., Yarden Y., 2007. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim. Biophys. Acta 1773: 1161–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko B. N., Hancock J. F., Kolch W., 2010. Signalling ballet in space and time. Nat. Rev. Mol. Cell Biol. 11: 414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan N. J., Greenblatt J. F., 2001. Characterization of a six-subunit Holo-Elongator complex required for the regulated expression of a group of genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 21: 8203–8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron S., Styles C. A., Fink G. R., 1994. Symmetric cell division in pseudohyphae of the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 5: 1003–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchin S., Vyas V. K., Carlson M., 2002. Snf1 protein kinase and the repressors Nrg1 and Nrg2 regulate FLO11, haploid invasive growth, and diploid pseudohyphal differentiation. Mol. Cell. Biol. 22: 3994–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. M., Mitchell A. P., 2003. The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 23: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. M., Xu W., Diamond A., Mitchell A. P., 2001. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 276: 1850–1856. [DOI] [PubMed] [Google Scholar]
- Lambrechts M. G., Bauer F. F., Marmur J., Pretorius I. S., 1996. Muc1, a mucin-like protein that is regulated by Mss10, is critical for pseudohyphal differentiation in yeast. Proc. Natl. Acad. Sci. USA 93: 8419–8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E., Wu C., Leeuw T., Fourest-Lieuvin A., Segall J. E., et al. , 1997. Functional characterization of the Cdc42p binding domain of yeast Ste20p protein kinase. EMBO J. 16: 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenssen E., Oberholzer U., Labarre J., De Virgilio C., Collart M. A., 2002. Saccharomyces cerevisiae Ccr4-not complex contributes to the control of Msn2p-dependent transcription by the Ras/cAMP pathway. Mol. Microbiol. 43: 1023–1037. [DOI] [PubMed] [Google Scholar]
- Lesage P., Yang X., Carlson M., 1996. Yeast SNF1 protein kinase interacts with SIP4, a C6 zinc cluster transcriptional activator: a new role for SNF1 in the glucose response. Mol. Cell. Biol. 16: 1921–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Wright S. J., Krystofova S., Park G., Borkovich K. A., 2007. Heterotrimeric G protein signaling in filamentous fungi. Annu. Rev. Microbiol. 61: 423–452. [DOI] [PubMed] [Google Scholar]
- Liu C., Yang Z., Yang J., Xia Z., Ao S., 2000. Regulation of the yeast transcriptional factor PHO2 activity by phosphorylation. J. Biol. Chem. 275: 31972–31978. [DOI] [PubMed] [Google Scholar]
- Liu H., Styles C. A., Fink G. R., 1996. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics 144: 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Butow R. A., 1999. A transcriptional switch in the expression of yeast tricarboxylic acid cycle genes in response to a reduction or loss of respiratory function. Mol. Cell. Biol. 19: 6720–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Butow R. A., 2006. Mitochondrial retrograde signaling. Annu. Rev. Genet. 40: 159–185. [DOI] [PubMed] [Google Scholar]
- Liu Z., Sekito T., Spirek M., Thornton J., Butow R. A., 2003. Retrograde signaling is regulated by the dynamic interaction between Rtg2p and Mks1p. Mol. Cell 12: 401–411. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Lo H., Kohler J. R., DiDomenico B., Loebenberg D., Cacciapuoti A., et al. , 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90: 939–949. [DOI] [PubMed] [Google Scholar]
- Lo W. S., Dranginis A. M., 1998. The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol. Biol. Cell 9: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., et al. , 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Lorenz M. C., Cutler N. S., Heitman J., 2000. Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol. Biol. Cell 11: 183–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani H. D., Fink G. R., 1997. Combinatorial control required for the specificity of yeast MAPK signaling. Science 275: 1314–1317. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Styles C. A., Fink G. R., 1997. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell 91: 673–684. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Galitski T., Lander E. S., Fink G. R., 1999. Effectors of a developmental mitogen-activated protein kinase cascade revealed by expression signatures of signaling mutants. Proc. Natl. Acad. Sci. USA 96: 12530–12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B., Kaiser C. A., 2002. Nitrogen regulation in Saccharomyces cerevisiae. Gene 290: 1–18. [DOI] [PubMed] [Google Scholar]
- Malathi K., Higaki K., Tinkelenberg A. H., Balderes D. A., Almanzar-Paramio D., et al. , 2004. Mutagenesis of the putative sterol-sensing domain of yeast Niemann Pick C-related protein reveals a primordial role in subcellular sphingolipid distribution. J. Cell Biol. 164: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheos D., Metodiev M., Muller E., Stone D., Rose M. D., 2004. Pheromone-induced polarization is dependent on the Fus3p MAPK acting through the formin Bni1p. J. Cell Biol. 165: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey G., Clay F. J., Kelsay K., Sprague G. F., Jr, 1987. Identification and regulation of a gene required for cell fusion during mating of the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 7: 2680–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney R. R., Schmidt M. C., 2001. Regulation of Snf1 kinase: activation requires phosphorylation of threonine 210 by an upstream kinase as well as a distinct step mediated by the Snf4 subunit. J. Biol. Chem. 276: 36460–36466. [DOI] [PubMed] [Google Scholar]
- McNeill H., Woodgett J. R., 2010. When pathways collide: collaboration and connivance among signalling proteins in development. Nat. Rev. Mol. Cell Biol. 11: 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measday V., Moore L., Retnakaran R., Lee J., Donoviel M., et al. , 1997. A family of cyclin-like proteins that interact with the Pho85 cyclin-dependent kinase. Mol. Cell. Biol. 17: 1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini L., Dudin O., Martin S. G., 2013. Mate and fuse: how yeast cells do it. Open Biol 3: 130008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. P., Lo R. S., Ben-Hur A., Desmarais C., Stagljar I., et al. , 2005. Large-scale identification of yeast integral membrane protein interactions. Proc. Natl. Acad. Sci. USA 102: 12123–12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat J., Andrews B., 2004. Late-G1 cyclin-CDK activity is essential for control of cell morphogenesis in budding yeast. Nat. Cell Biol. 6: 59–66. [DOI] [PubMed] [Google Scholar]
- Mosch H. U., Roberts R. L., Fink G. R., 1996. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93: 5352–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosch H. U., Kubler E., Krappmann S., Fink G. R., Braus G. H., 1999. Crosstalk between the Ras2p-controlled mitogen-activated protein kinase and cAMP Pathways during invasive growth of Saccharomyces cerevisiae. Mol. Biol. Cell 10: 1325–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman A. M., 2011. Sporulation in the budding yeast Saccharomyces cerevisiae. Genetics 189: 737–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile C. J., Nett J. E., Andes D. R., Mitchell A. P., 2006. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell 5: 1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill E., Kaffman A., Jolly E. R., O’Shea E. K., 1996. Regulation of PH04 nuclear localization by the PH080–PH085 cyclin-CDK complex. Science 271: 209–212. [DOI] [PubMed] [Google Scholar]
- O’Rourke S. M., Herskowitz I., 1998. The Hog1 MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes Dev. 12: 2874–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek S. P., Parikh A. S., Kron S. J., 2000. Genetic analysis reveals that FLO11 upregulation and cell polarization independently regulate invasive growth in Saccharomyces cerevisiae. Genetics 156: 1005–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Heitman J., 1999. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Cell. Biol. 19: 4874–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Heitman J., 2002. Protein kinase A operates a molecular switch that governs yeast pseudohyphal differentiation. Mol. Cell. Biol. 22: 3981–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. O., Bi E., Pringle J. R., Herskowitz I., 1997. Two active states of the Ras-related Bud1/Rsr1 protein bind to different effectors to determine yeast cell polarity. Proc. Natl. Acad. Sci. USA 94: 4463–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. O., Kang P. J., Rachfal A. W., 2002. Localization of the Rsr1/Bud1 GTPase involved in selection of a proper growth site in yeast. J. Biol. Chem. 277: 26721–26724. [DOI] [PubMed] [Google Scholar]
- Peter M., Neiman A. M., Park H. O., van Lohuizen M., Herskowitz I., 1996. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. EMBO J. 15: 7046–7059. [PMC free article] [PubMed] [Google Scholar]
- Petrakis T. G., Wittschieben B. O., Svejstrup J. Q., 2004. Molecular architecture, structure-function relationship, and importance of the Elp3 subunit for the RNA binding of holo-elongator. J. Biol. Chem. 279: 32087–32092. [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W., 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitoniak A., Birkaya B., Dionne H. M., Vadaie N., Cullen P. J., 2009. The signaling mucins Msb2 and Hkr1 differentially regulate the filamentation mitogen-activated protein kinase pathway and contribute to a multimodal response. Mol. Biol. Cell 20: 3101–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pray-Grant M. G., Schieltz D., McMahon S. J., Wood J. M., Kennedy E. L., et al. , 2002. The novel SLIK histone acetyltransferase complex functions in the yeast retrograde response pathway. Mol. Cell. Biol. 22: 8774–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D., Bretscher A., 2000. Polarization of cell growth in yeast. J. Cell Sci. 113: 365–375. [DOI] [PubMed] [Google Scholar]
- Raman M., Chen W., Cobb M. H., 2007. Differential regulation and properties of MAPKs. Oncogene 26: 3100–3112. [DOI] [PubMed] [Google Scholar]
- Reynolds T. B., 2006. The Opi1p transcription factor affects expression of FLO11, mat formation, and invasive growth in Saccharomyces cerevisiae. Eukaryot. Cell 5: 1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds T. B., Fink G. R., 2001. Bakers’ yeast, a model for fungal biofilm formation. Science 291: 878–881. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Nelson B., Marton M. J., Stoughton R., Meyer M. R., et al. , 2000. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science 287: 873–880. [DOI] [PubMed] [Google Scholar]
- Roberts R. L., Fink G. R., 1994. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 8: 2974–2985. [DOI] [PubMed] [Google Scholar]
- Robertson L. S., Fink G. R., 1998. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc. Natl. Acad. Sci. USA 95: 13783–13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Winston F., Hieter P., 1990. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Rothermel B. A., Shyjan A. W., Etheredge J. L., Butow R. A., 1995. Transactivation by Rtg1p, a basic helix-loop-helix protein that functions in communication between mitochondria and the nucleus in yeast. J. Biol. Chem. 270: 29476–29482. [DOI] [PubMed] [Google Scholar]
- Rupp S., Summers E., Lo H. J., Madhani H. D., Fink G. R., 1999. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 18: 1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan O., Shapiro R. S., Kurat C. F., Mayhew D., Baryshnikova A., et al. , 2012. Global gene deletion analysis exploring yeast filamentous growth. Science 337: 1353–1356. [DOI] [PubMed] [Google Scholar]
- Saito H., 2010. Regulation of cross-talk in yeast MAPK signaling pathways. Curr. Opin. Microbiol. 13: 677–683. [DOI] [PubMed] [Google Scholar]
- Saito H., Posas F., 2012. Response to hyperosmotic stress. Genetics 192: 289–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman L. R., Caruso C., Page N., Pringle J. R., 2002. Role of cell-cycle regulated expression in the localization of spatial landmark proteins in yeast. J. Cell Biol. 156: 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Beck T., Koller A., Kunz J., Hall M. N., 1998. The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 17: 6924–6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekito T., Liu Z., Thornton J., Butow R. A., 2002. RTG-dependent mitochondria-to-nucleus signaling is regulated by MKS1 and is linked to formation of yeast prion [URE3] Mol. Biol. Cell 13: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R. S., Sellam A., Tebbji F., Whiteway M., Nantel A., et al. , 2012. Pho85, Pcl1, and Hms1 signaling governs Candida albicans morphogenesis induced by high temperature or Hsp90 compromise. Curr. Biol. 22: 461–470. [DOI] [PubMed] [Google Scholar]
- Shemer R., Meimoun A., Holtzman T., Kornitzer D., 2002. Regulation of the transcription factor Gcn4 by Pho85 cyclin Pcl5. Mol. Cell. Biol. 22: 5395–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu Y. J., Barral Y., Snyder M., 2000. Polarized growth controls cell shape and bipolar bud site selection in Saccharomyces cerevisiae. Mol. Cell. Biol. 20: 5235–5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively C. A., Eckwahl M. J., Dobry C. J., Mellacheruvu D., Nesvizhskii A., et al. , 2013. Genetic networks inducing invasive growth in Saccharomyces cerevisiae identified through systematic genome-wide overexpression. Genetics 193: 1297–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn K., Schwenk J., Urban C., Lechner J., Schweikert M., et al. , 2006. Getting in touch with Candida albicans: the cell wall of a fungal pathogen. Curr. Drug Targets 7: 505–512. [DOI] [PubMed] [Google Scholar]
- Stevenson B. J., Rhodes N., Errede B., Sprague G. F., Jr, 1992. Constitutive mutants of the protein kinase STE11 activate the yeast pheromone response pathway in the absence of the G protein. Genes Dev. 6: 1293–1304. [DOI] [PubMed] [Google Scholar]
- Svejstrup J. Q., 2007. Elongator complex: How many roles does it play? Curr. Opin. Cell Biol. 19: 331–336. [DOI] [PubMed] [Google Scholar]
- Tatebayashi K., Yamamoto K., Tanaka K., Tomida T., Maruoka T., et al. , 2006. Adaptor functions of Cdc42, Ste50, and Sho1 in the yeast osmoregulatory HOG MAPK pathway. EMBO J. 25: 3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., et al. , 1985. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell 40: 27–36. [DOI] [PubMed] [Google Scholar]
- Uetz P., Giot L., Cagney G., Mansfield T. A., Judson R. S., et al. , 2000. A comprehensive analysis of protein–protein interactions in Saccharomyces cerevisiae. Nature 403: 623–627. [DOI] [PubMed] [Google Scholar]
- Vadaie N., Dionne H., Akajagbor D. S., Nickerson S. R., Krysan D. J., et al. , 2008. Cleavage of the signaling mucin Msb2 by the aspartyl protease Yps1 is required for MAPK activation in yeast. J. Cell Biol. 181: 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstrepen K. J., Klis F. M., 2006. Flocculation, adhesion and biofilm formation in yeasts. Mol. Microbiol. 60: 5–15. [DOI] [PubMed] [Google Scholar]
- Voordeckers K., De Maeyer D., van der Zande E., Vinces M. D., Meert W., et al. , 2012. Identification of a complex genetic network underlying Saccharomyces cerevisiae colony morphology. Mol. Microbiol. 86: 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Nebreda A. R., 2009. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9: 537–549. [DOI] [PubMed] [Google Scholar]
- Wendland J., 2001. Comparison of morphogenetic networks of filamentous fungi and yeast. Fungal Genet. Biol. 34: 63–82. [DOI] [PubMed] [Google Scholar]
- White M. J., Hirsch J. P., Henry S. A., 1991. The OPI1 gene of Saccharomyces cerevisiae, a negative regulator of phospholipid biosynthesis, encodes a protein containing polyglutamine tracts and a leucine zipper. J. Biol. Chem. 266: 863–872. [PubMed] [Google Scholar]
- Winkler G. S., Petrakis T. G., Ethelberg S., Tokunga M., Erdjument-Bromage H., et al. , 2001. RNA polymerase II elongator holoenzyme is composed of two discrete subcomplexes. J. Biol. Chem. 276: 32743–32749. [DOI] [PubMed] [Google Scholar]
- Woods A., Munday M. R., Scott J., Yang X., Carlson M., et al. , 1994. Yeast SNF1 is functionally related to mammalian AMP-acitvate protein kinase and regulates acetyl-CoA carboxylase in vivo. J. Biol. Chem. 269: 19509–19515. [PubMed] [Google Scholar]
- Wu C., Jansen G., Zhang J., Thomas D. Y., Whiteway M., 2006. Adaptor protein Ste50p links the Ste11p MEKK to the HOG pathway through plasma membrane association. Genes Dev. 20: 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Shively C. A., Jin R., Eckwahl M. J., Dobry C. J., et al. , 2010. A profile of differentially abundant proteins at the yeast cell periphery during pseudohyphal growth. J. Biol. Chem. 285: 15476–15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Tatebayashi K., Tanaka K., Saito H., 2010. Dynamic control of yeast MAP kinase network by induced association and dissociation between the Ste50 scaffold and the Opy2 membrane anchor. Mol. Cell 40: 87–98. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Tatebayashi K., Yamamoto K., Saito H., 2009. Glycosylation defects activate filamentous growth Kss1 MAPK and inhibit osmoregulatory Hog1 MAPK. Embo. J. 28: 1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlinger J., Simon I., Harbison C. T., Hannett N. M., Volkert T. L., et al. , 2003. Program-specific distribution of a transcription factor dependent on partner transcription factor and MAPK signaling. Cell 113: 395–404. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kweon H. K., Shively C., Kumar A., Andrews P. C., 2013. Towards systematic discovery of signaling networks in budding yeast filamentous growth stress response using interventional phosphorylation data. PLOS Comput. Biol. 9: e1003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Mehrabi R., Xu J. R., 2007. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell 6: 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.