Abstract
One major hurdle to the success of adoptive T-cell therapy is the identification of antigens that permit effective targeting of tumors in the absence of toxicities to essential organs. Previous work has demonstrated that T cells engineered to express chimeric antigen receptors (CAR-T cells) targeting the murine homolog of the colorectal cancer antigen GUCY2C treat established colorectal cancer metastases, without toxicity to the normal GUCY2C-expressing intestinal epithelium, reflecting structural compartmentalization of endogenous GUCY2C to apical membranes comprising the intestinal lumen. Here, we examined the utility of a human-specific, GUCY2C-directed scFv as the basis for a CAR construct targeting human GUCY2C-expressing metastases. Human GUCY2C-targeted murine CAR-T cells promoted antigen-dependent T-cell activation quantified by activation marker upregulation, cytokine production, and killing of GUCY2C-expressing, but not GUCY2C-deficient, cancer cells in vitro. GUCY2C CAR-T cells provided long-term protection against lung metastases of murine colorectal cancer cells engineered to express human GUCY2C in a syngeneic mouse model. GUCY2C murine CAR-T cells recognized and killed human colorectal cancer cells endogenously expressing GUCY2C, providing durable survival in a human xenograft model in immunodeficient mice. Thus, we have identified a human GUCY2C-specific CAR-T cell therapy approach that may be developed for the treatment of GUCY2C-expressing metastatic colorectal cancer.
Keywords: adoptive immunotherapy, chimeric antigen receptors, colorectal cancer, guanylyl cyclase c
Introduction
Chimeric antigen receptor (CAR)-expressing T cells are effective in patients with advanced B-cell malignancies and neuroblastoma (1–3). However, the clinical utility of adoptively transferred T cells has been limited in patients with colorectal cancer, reflecting antigen “on-target, off-tumor” toxicities limiting treatment options (4,5). Guanylyl cyclase C (GUCY2C) is a membrane-bound receptor that produces the second messenger cGMP following activation by its hormone ligands guanylin or uroguanylin, regulating intestinal homeostasis, tumorigenesis, and obesity (6). GUCY2C cell surface expression is confined to luminal surfaces of the intestinal epithelium and a subset of hypothalamic neurons (7). Its expression is maintained in >95% of colorectal cancer metastases and it is ectopically expressed in tumors that evolve from intestinal metaplasia, including esophageal, gastric, and pancreatic cancers (8,9). The inaccessibility of GUCY2C in the apical membranes of polarized epithelial tissue (10), due to subcellular restriction of GUCY2C, creates a therapeutic opportunity to target metastatic lesions of colorectal origin which have lost apical-basolateral polarization (11), without concomitant intestinal toxicity. Previously, we demonstrated in a syngeneic, immunocompetent mouse model that CAR-T cells targeting murine GUCY2C were effective against colorectal cancer metastatic to lung in the absence of intestinal toxicities (12). Similarly, other GUCY2C-targeted therapeutics, including antibody-drug conjugates (13) and vaccines (14,15), are safe in preclinical animal models, and therapeutic regimens utilizing these platforms are in clinical trials for metastatic esophageal, gastric, pancreatic, and colorectal cancers (NCT02202759, NCT02202785, NCT01972737). The safety of these therapeutic regimens, in the context of GUCY2C expression across the rostral-caudal axis of intestine, reflects compartmentalized expression of GUCY2C, enriched in apical, but limited in basolateral, membranes of epithelial cells (16–19). Systemic radiolabeled imaging agents conjugated to GUCY2C ligand target GUCY2C-expressing metastases without localizing in intestine (10), confirming the mucosal compartmentalization of the receptor. Tumors express up to 10-fold greater amounts of GUCY2C, compared to normal epithelial cells (9,20), potentially creating a quantitative therapeutic window to discriminate receptor overexpressing tumors from intestinal epithelium (21) with low/absent GUCY2C in basolateral membranes (19).
Here, we identified a human GUCY2C-targeted CAR that could potentially be employed in patients with GUCY2C-expressing gastrointestinal malignancies. This CAR induced antigen-dependent T-cell activation, cytokine production, and cytolytic activity. Human GUCY2C-targeted CAR-T cells were effective against metastatic tumors in immunocompetent, syngeneic mouse models, as well as xenograft models of human colorectal cancer.
Materials and Methods
Cell lines and reagents
CT26 and β-galactosidase–expressing CT26.CL25 mouse colorectal cancer cell lines and the human colorectal cancer cell lines T84 and SW480 were obtained from ATCC and large stocks of low-passage cells were cryopreserved. Cells were authenticated by the original suppliers and routinely authenticated by morphology, growth, antibiotic resistance (where appropriate), appropriate GUCY2C and β-galactosidase expression, and pattern of metastasis in vivo and routinely screened for mycoplasma using the Universal Mycoplasma Detection Kit (ATCC, Cat. No. 30-1012K). Before injection into mice, cells were routinely cultured for <2 weeks. The gene encoding human GUCY2C was codon-optimized (Supplementary Fig. S1) and synthesized (Gene Art, Life Technologies) and cloned into the retroviral construct pMSCVpuro (Clontech). CT26.hGUCY2C and CT26.CL25.hGUCY2C were generated by transducing CT26 and CT26.CL25 cells with retroviral supernatants encoding hGUCY2C, followed by selection with puromycin. Retroviral supernatants were produced by transfecting the Phoenix-Eco retroviral packaging cell line (Gary Nolan, Stanford University) with pMSCV-Puro (Clontech) or hGUCY2C-pMSCV-Puro and the pCL-Eco (Imgenex) retroviral packaging vector (12). Luciferase-containing T84.fLuc cells were generated by transduction with lentiviral supernatants generated by transfecting 293FT cells (Invitrogen) with pLenti4-V5-GW-luciferase.puro (kindly provided by Andrew Aplin, Thomas Jefferson University) and the ViraPower Lentiviral Packaging Mix (Invitrogen) according in manufacturer instructions, followed by selection in puromycin. The single chain variable fragment (scFv) from the human GUCY2C-specific antibody 5F9 (Supplementary Fig. S2) was cloned into the pFUSE-rIgG-Fc2 (IL2ss) plasmid (Invivogen), producing a 5F9 scFv fusion protein with rabbit Fc (5F9-rFc). 5F9-rFc was collected in supernatants of transfected 293F cells (Life Technologies), titrated in ELISA plates (Nunc-Immuno PolySorp) coated with BSA or recombinant 6xHis-tagged hGUCY2C extracellular domain (6xHis-hGUCY2CECD) protein purified under contract from HEK293-6E cells by GenScript and detected with HRP-conjugated goat anti-rabbit (Jackson ImmunoResearch). For flow cytometry, cells were stained with 5F9-rFc or control supernatants from un-transfected 293F cells diluted in FACS buffer (1% heat-inactivated FBS in PBS), followed by secondary Alexa Fluor 488–conjugated anti-rabbit (Life Technologies) in FACS buffer. Cells were fixed with 2% paraformaldehyde (PFA; Affymetrix) and analyzed using the BD LSR II flow cytometer and FlowJo v10 software (Tree Star).
Murine CAR-T Cell Generation
Murine CAR components were employed to produce a third-generation, codon-optimized retroviral CAR construct as previously described (12). A codon-optimized scFv sequence derived from the 5F9 human GUCY2C-specific antibody (Supplementary Fig. S2) was cloned into a CAR construct containing murine sequences of the BiP signal peptide, CD8α hinge region, CD28 transmembrane and intracellular domains, and 4-1BB (CD137) and CD3ζ intracellular domains, producing the 5F9.m28BBz CAR construct (Supplementary Fig. S3). CARs derived from the human ERBB2 (Her2)-specific antibody 4D5 or mouse CD19-specific antibody 1D3 (Supplementary Fig. S2) were used as controls as indicated (Control m28BBz). CARs were subcloned into the pMSCV-IRES-GFP (pMIG) retroviral vector (Addgene # 27490). The Phoenix-Eco retroviral packaging cell line (Gary Nolan, Stanford University) was transfected with CAR-pMIG vectors and the pCL-Eco retroviral packaging vector (Imgenex) using the Calcium Phosphate ProfectionR Mammalian Transfection System (Promega). Retrovirus-containing supernatants were collected 48 hours later, filtered through 0.45 μM filters, and aliquots were frozen at −80°C.
Murine CD8+ T cells were negatively selected from BALB/c splenocytes using the CD8α+ T cell Isolation Kit II and LS magnetic columns (Miltenyi Biotec). CD8+ T cells were subsequently stimulated with anti-CD3/anti-CD28–coated beads (T Cell Activation/Expansion Kit, Miltenyi Biotec) at a 1:1 bead:cell ratio at 1x106 cells/mL in cRPMI with recombinant human IL2 (100 U/mL; NCI Repository). The day following stimulation, half of the culture media was carefully replaced with an equal volume of thawed retroviral supernatant in the presence of polybrene (8 μg/mL; Millipore). Spinoculation was performed at room temperature for 90 minutes at 2500 rpm followed by incubation at 37°C for 2.5 hours, at which point cells were pelleted and resuspended in fresh media containing IL2 (100 U/mL). T cells were expanded for 7–10 days by daily dilution to 1x106 cells/mL with fresh cRPMI and IL2 at which point they were used for functional assays.
Human CAR-T Cell Generation
For studies with human T cells, PBMCs were collected from consenting volunteers in accordance with regulatory and institutional requirements. MACS (Stemcell Technologies) purified CD8+ T cells were negatively selected from individual normal healthy donor whole blood at >90% purity. CAR domains employing human sequences were used to produce a third-generation, codon-optimized retroviral CAR construct containing the 5F9 human GUCY2C-specific scFv and human sequences of the GM-CSF signal peptide, CD8α hinge region, CD28 transmembrane and intracellular domains, and 4-1BB (CD137) and CD3ζ intracellular domains producing 5F9.h28BBz (Supplementary Fig. S4). CAR-encoding amphotropic γ-retrovirus production was similar to that with murine T cells, but replaced pCL-Eco with the pCL-Ampho packaging plasmid (Imgenex). Retroviral transduction occurred on day 3 or 4 post-activation with ImmunoCult CD3/CD28 Activator (Stem Cell Technologies). Cells underwent flow sorting for GFP-enrichment on day 7, followed by experimental use on day 10. Throughout the duration in culture, human CD8+ T cells were maintained in ImmunoCult-XF media (Stemcell Technologies) supplemented with 100 U/mL recombinant, human IL2 (NCI Repository).
CAR Surface Detection
CAR-transduced T cells were stained with the LIVE/DEAD Fixable Aqua Dead Cell Stain kit (Invitrogen) in PBS, labeled with varying concentrations of 6xHis-hGUCY2CECD for 1 hour in PBS 0.5% BSA, stained with anti-5xHis-Alexa Fluor 647 conjugate (Qiagen) and anti-CD8b-PE (clone H35.17.2, BD Biosciences) for 1 hour in PBS 0.5% BSA, fixed with 2% PFA and analyzed using the BD LSR II flow cytometer and FlowJo software v10 (Tree Star). hGUCY2C binding was determined by mean fluorescence intensity of Alexa Fluor 647 on live CD8+ CAR+ (GFP+) cells. Non-linear regression analysis (GraphPad Prism v6) was used to determine the Kav and Bmax of GUCY2C-CAR binding.
Mouse T-cell Phenotyping, Activation Markers, and Intracellular Cytokine Staining
For phenotyping, 1x106 non-transduced or CAR-transduced mouse T cells were stained with LIVE/DEAD Fixable Aqua Dead Cell Stain kit (Invitrogen) in PBS and subsequently stained for surface markers using anti-CD8α–BV570 (clone RPA-T8; Biolegend), anti-CD45RA–PerCP-Cy5.5 (clone 14.8; BD Biosciences), and anti-CD62L–PE-Cy7 (clone MEL-14; BD Biosciences) for 30 minutes in PBS 0.5% BSA. Cells were subsequently fixed and permeabilized (BD Cytofix/Cytoperm Kit; BD Biosciences) with Cytofix/Cytoperm buffer for 20 minutes at 4°C and stained for intracellular GFP (anti-GFP–Alexa Fluor 488; Invitrogen) for 45 minutes in Perm/Wash buffer to identify CAR-transduced cells. The following subsets were then quantified based on CD45RA and CD62L staining: Tn/scm (naïve or T memory stem cells; CD62L+CD45RA+), Tcm (central memory T cells; CD62L+CD45RA−), Tem (effector memory T cells; CD62L−CD45RA−), and Temra (effector memory T cells expressing CD45RA; CD62L−CD45RA+).
For activation marker and cytokine analysis, 1x106 CAR-transduced mouse T cells were stimulated for 6 hours in tissue culture plates previously coated with 1 μg/mL GUCY2C in PBS overnight at 4°C or in tissue culture plates containing 1x106 CT26 or CT26.hGUCY2C cells. As a positive control, CAR-T cells were incubated for 6 hours with 1X Cell Stimulation Cocktail (PMA/Ionomycin, eBioscience). Incubation included 1X Protein Transport Inhibitor Cocktail (eBioscience) when assessing intracellular cytokines. Cells were stained with LIVE/DEAD Fixable Aqua Dead Cell Stain kit (Invitrogen) and subsequently stained for surface markers using anti-CD8α–PerCP-Cy5.5 (clone 53.6–7; BD Biosciences), anti-CD69–PE (clone H1.2F3; BD Biosciences), anti-CD25–PE (clone PC61.5, eBioscience), and anti-CD44–APC (clone IM7; Biolegend). Intracellular cytokine staining was performed using the BD Cytofix/Cytoperm Kit (BD Biosciences) and staining with anti-GFP–Alexa488 (Invitrogen), anti-IFNγ–APC-Cy7 (clone XMG1.2; BD Biosciences), anti-TNFα–PE-Cy7 (clone MP6-XT22; BD Biosciences), anti-IL2–APC (clone JES6-5H4; BD Biosciences) and αMIP1α–PE (clone 39624; R&D Systems). Cells were fixed in 2% PFA and analyzed on a BD LSR II flow cytometer. Analyses were performed using FlowJo v10 software (Tree Star).
Human T-cell Activation Marker and Intracellular Cytokine Staining
For activation marker and cytokine analysis, 1x106 human GUCY2C-directed CAR-transduced human T cells were stimulated for 6 hours in tissue culture plates coated overnight at 4°C with 10 μg/mL human GUCY2C or BSA control antigen in PBS or with 1X Cell Stimulation Cocktail (PMA/Ionomycin, eBioscience) added at the time of plating CAR-T cells. All conditions included 1X Protein Transport Inhibitor Cocktail (eBioscience) at the beginning of the incubation period. Cells were stained with LIVE/DEAD Fixable Aqua Dead Cell Stain kit (Invitrogen) in PBS for 10 minutes and subsequently stained for surface markers using anti-CD8–Qdot 800 (clone 3B5, Invitrogen) and anti-CD69–APC (clone L78, BD Biosciences) in PBS 0.5% BSA for 25 minutes. Intracellular cytokine staining was performed using the BD Cytofix/Cytoperm Kit (BD Biosciences) consisting of fixation with Cytofix/Cytoperm buffer for 20 minutes and staining with anti-GFP–Alexa Fluor 488 (Invitrogen), anti-IFNγ–BV605 (clone 4S.B3; BioLegend), anti-TNFα–PerCP-Cy5.5 (clone Mab11; BD Biosciences), and anti-IL2–PE (clone MQ1-17H12; BD Biosciences) in BD perm wash buffer for 45 minutes. Cells were fixed in 2% PFA and analyzed on a BD LSR II flow cytometer. Analyses were performed using FlowJo v10 software (Tree Star).
T-Cell Cytotoxicity Assays
The xCELLigence real-time, cell-mediated cytotoxicity system (Acea Biosciences Inc.) was utilized for assessment of CAR-T cell–mediated cytotoxicity as previously described (12). Briefly, 1x104 CT26 or CT26.hGUCY2C or 2.5x104 T84 or SW480 cancer cell targets were plated in 150 μL of growth medium in each well of an E-Plate 16 (Acea Biosciences) and grown overnight in a 37°C incubator, quantifying electrical impedance every 15 minutes using the RTCA DP Analyzer system and RTCA software version 2.0 (Acea Biosciences Inc.). Approximately 24 hours later for mouse and 6 hours for human T cell experiments, 50 μL of CAR-T cells were added (5:1 E:T ratio for mouse T cells or 10:1 E:T ratio for human T cells), or 50 μL of media or 10% Triton-X 100 (Fisher) was added for a final (v/v) of 2.5% Triton-X 100 as negative and positive controls, respectively. Cell-mediated killing was quantified over the next 10–20 hours, reading electrical impedance every 15 minutes. Percent specific lysis values were calculated using GraphPad Prism Software v6 for each replicate at each time point, using impedance values following the addition of media and Triton-X 100 for normalization (0% and 100% specific lysis, respectively).
Alternatively, the β-gal release T-cell cytotoxicity assay utilized CT26 cancer cell targets expressing β-galactosidase (CT26.CL25). Cancer cell targets were plated at 2x105 cells/well in a 96-well plate and incubated with increasing effector CAR-T cell to cancer cell target ratios for 4 hours at 37°C. Released β-galactosidase was measured in the media using the Galacto-Light Plus System (Applied Biosystems, Carlsbad, California). Maximum release was determined from supernatants of cells that were lysed with supplied lysis buffer. % specific lysis = [(experimental release - spontaneous release)/(maximum release - spontaneous release)] x 100.
Metastatic Tumor Models
BALB/c mice and NSG (NOD-scid IL2Rγnull) mice were obtained from the NCI Animal Production Program (Frederick, MD) and Jackson Labs (Bar Harbor, ME), respectively. Animal protocols were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee. In syngeneic mouse models, BALB/c mice were injected with 5x105 CT26.hGUCY2C cells in 100 μL of PBS by tail vein injection to establish lung metastases. On indicated days, mice received a non-myeloablative dose of 5 Gy total body irradiation in a PanTak, 310kVe x-ray machine (12). Mice received the indicated dose of CAR-T cells produced from CD8+ BALB/c T cells in 100 μL of PBS by tail vein at the indicated time points. Mice were followed for survival or sacrificed on day 18 after cancer cell injection and lungs were stained with India Ink and fixed in Fekete’s solution for tumor enumeration (22).
For re-challenge experiments, naïve mice or mice cleared of established tumors by CAR-T cells (referred to as “surviving mice”) received one dose of 5x105 CT26 or CT26.hGUCY2C via tail vein injection. Surviving mice were initially challenged 16–40 weeks prior to the re-challenge experiment.
In human tumor xenograft models, NSG (23) mice (JAX stock #005557) were injected with 2.5x106 T84.fLuc cells in 100 μL PBS via intraperitoneal injection. Mice received a dose of 107 total (not sorted on CAR+) T cells produced from CD8+ BALB/c T cells in 100 μL PBS via intraperitoneal injection on day 14 after cancer cell inoculation. Tumor growth was monitored weekly by subcutaneous injection of a 250 μL solution of 15 mg/ml D-luciferin potassium salt (Gold Biotechnologies) in PBS and imaging after 8 minutes of exposure using the Caliper IVIS Lumina-XR imaging station (Perkin Elmer). Total radiance (photons/second) was quantified using Living Image In Vivo Imaging Software (Perkin Elmer).
Results and Discussion
hGUCY2C CAR-T cells
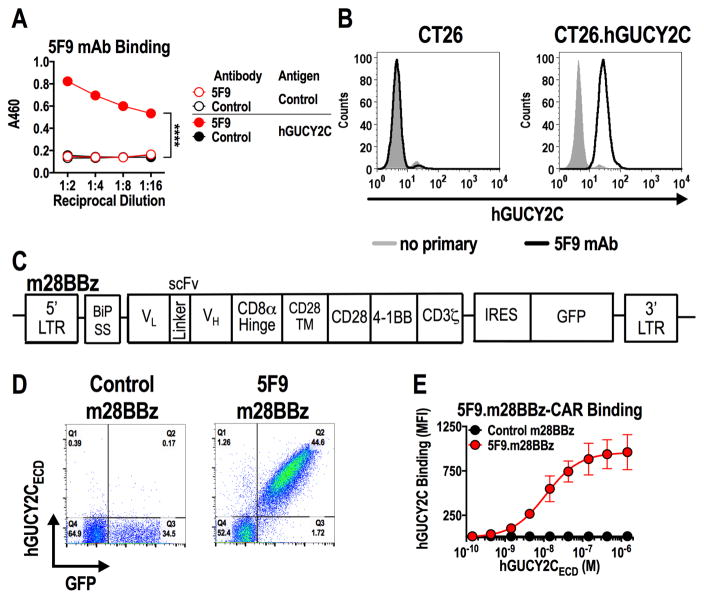
A recombinant antibody (clone 5F9) specific for human GUCY2C (hGUCY2C) bound to purified hGUCY2C extracellular domain (Fig. 1A) and murine CT26 colorectal cancer cells engineered to express hGUCY2C, but not hGUCY2C-deficient CT26 cancer cells (Fig. 1B). The 5F9 scFv was used to generate a third-generation murine CAR construct (5F9.m28BBz) containing the BiP signal sequence, CD8α hinge region, and intracellular CD28, 4-1BB, and CD3ζ signaling moieties and inserted into a retroviral construct (Fig. 1C). Retroviruses encoding control m28BBz or 5F9.m28BBz CARs were used to transduce murine T cells with ~35–45% transduction efficiency, quantified by a GFP transduction marker (Fig. 1D). hGUCY2C-binding avidity (Kav = 11.2 nM) and CAR expression (Bmax = 957.8 MFI), quantified by incubating CAR-T cells with increasing concentrations of purified 6xHis-tagged hGUCY2CECD followed by detection with labeled α5xHis antibody and assessment by flow cytometry, was comparable to CARs that exhibited functional reactivity to mouse GUCY2C (12) (Fig. 1D and E, Supplementary Fig. S5).
Figure 1. Generation of human GUCY2C-specific CAR-T cells.
(A) Recombinant 5F9 antibody was assessed by ELISA for specific binding to hGUCY2CECD or BSA (negative control) plated at 1 μg/mL. Two-way ANOVA; ****p<0.0001. (B) Flow cytometry analysis was performed on parental CT26 mouse colorectal cancer cells or CT26 cells engineered to express hGUCY2C (CT26.hGUCY2C) and stained with 5F9 antibody. (C) Schematic of the third-generation murine CAR construct containing murine sequences of the BiP signal sequence, 5F9 scFv, CD8α hinge region, the transmembrane and intracellular domain of CD28, the intracellular domain of 4-1BB (CD137), and the intracellular domain of CD3ζ (5F9.m28BBz). The CAR construct was inserted into the MSCV retroviral plasmid pMIG upstream of an IRES-GFP marker. (D) Murine CD8+ T cells transduced with a retrovirus containing a control (1D3.m28BBz) CAR or CAR derived from the 5F9 antibody (5F9.m28BBz) were labeled with purified 6xHis-hGUCY2CECD (10 μg/mL), detected with anti-5xHis-Alexa Fluor 647 conjugate. Flow plots were gated on live CD8+ cells. (E) 6xHis-hGUCY2CECD binding curves for 5F9-derived or control (1D3) CARs, gated on live CD8+GFP+ cells (Supplementary Fig. S5). Combined from 3 independent experiments.
hGUCY2C CAR mediates T-cell activation and effector function
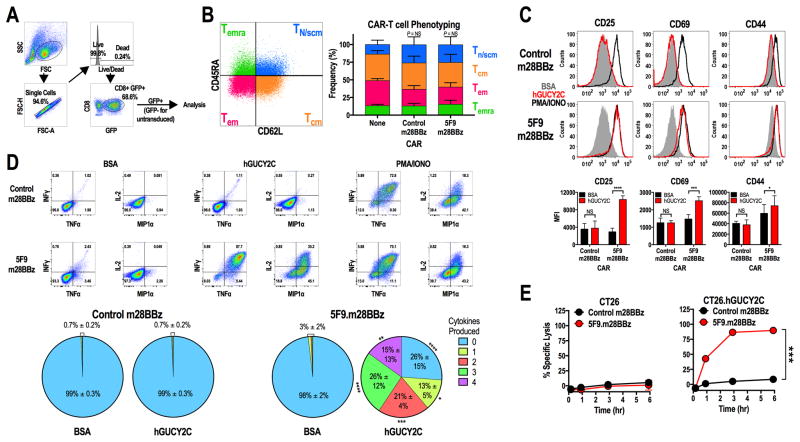
Transduction of purified mouse CD8+ T cells with control m28BBz or hGUCY2C-specific 5F9.m28BBz CAR constructs had no impact on T-cell phenotype compared to non-transduced cells (Fig. 2B), producing a mixture of memory and effector phenotypes [Tn/scm (CD62L+CD45RA+), Tcm (CD62L+CD45RA−), Tem (CD62L−CD45RA−), and Temra (CD62L−CD45RA+)] similar to other CAR constructs in CAR-T cells produced in the presence of IL2 (24). hGUCY2C-specific, but not control, CAR-T cells upregulated the activation markers CD25, CD69, and CD44 (Fig. 2C) and produced the effector cytokines IFNγ, TNFα, IL2, and MIP1α (Fig. 2D) when stimulated with immobilized hGUCY2CECD protein or CT26.hGUCY2C cells (Supplementary Fig. S6 and S7). Activation marker and cytokine responses were absent when 5F9.m28BBz CAR-T cells were stimulated with BSA or hGUCY2C-deficient CT26 cells, confirming that T-cell activation by the 5F9.m28BBz CAR is antigen-dependent (Fig. 2C–D, Supplementary Fig. S6 and S7). Although 5F9.m28BBz CAR-T cells were inactive against hGUCY2C-deficient CT26 cells in vitro (Fig. 2E), they exhibited time-dependent killing of CT26.hGUCY2C cells, quantified by employing an electrical impedance assay (Fig. 2E) and confirmed in a β-galactosidase release T-cell cytotoxicity assay (Supplementary Fig. S8).
Figure 2. hGUCY2C-specific CARs mediate antigen-dependent T-cell activation and effector functions.
(A–E) Murine CD8+ T cells were left non-transduced (None) or transduced with control 1D3.m28BBz or 5F9.m28BBz CAR constructs as indicated. (A) Gating strategy for all analyses in B–D. (B) Representative CAR-T cell phenotyping plot based on CD45RA and CD62L. Two-way ANOVA; NS: not significant; Bars: mean ± SD from 2–3 independent experiments; Tn/scm: naïve or T memory stem cells; Tcm: central memory T cells; Tem: effector memory T cells; Temra: effector memory T cells expressing CD45RA. (C–D) 106 CAR-T cells were stimulated for 6 hours with plate-coated antigen (BSA or hGUCY2C) or PMA and ionomycin (PMA/IONO). T-cell activation markers (CD25, CD69, or CD44) and intracellular cytokine production (IFNγ, TNFα, IL2, and MIP1α) were then quantified by flow cytometry. Graphs indicate the mean ± SD (C) activation marker upregulation (MFI) and (D) polyfunctional cytokine production (% of CAR+ cells) from 3 independent experiments. (E) Parental CT26 or CT26.hGUCY2C mouse colorectal cancer cells in an E-Plate were treated with CAR-T cells (5:1 E:T ratio), media, or 10% Triton-X 100 (Triton), and the relative electrical impedance was quantified every 15 minutes for 10 hours to quantify cancer cell death (normalized to time=0). Percent specific lysis values were calculated using impedance values following the addition of media and Triton for normalization (0% and 100% specific lysis, respectively). Two-way ANOVA, B–E; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
hGUCY2C CAR-T cells oppose metastatic colorectal cancer
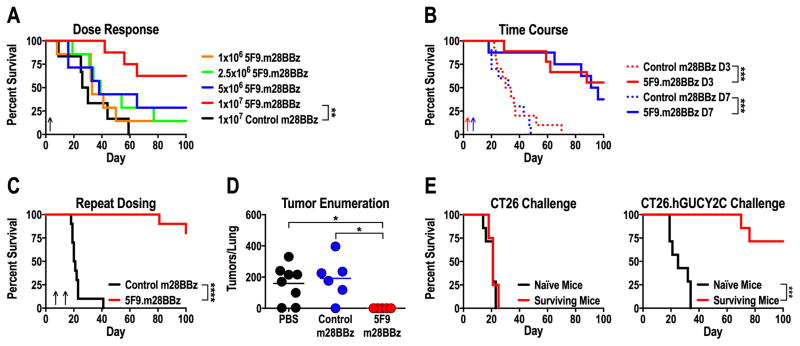
The endogenous immune system can induce immunosuppression in the tumor microenvironment and compete with adoptively transferred T cells for resources necessary for long-term persistence (25). In that context, lymphodepletive conditioning regimens, such as low-dose total body irradiation (TBI) or chemotherapies, enhance the efficacy of adoptively transferred T cells by eliminating immunosuppressive cells and reducing competition for homeostatic cytokines, including IL7 and IL15 (25,26). Here, we utilized an immunocompetent mouse model and a non-myeloablative dose of 5 Gy total body irradiation (TBI) to mimic clinical treatment regimens. Immunocompetent BALB/c mice received CT26.hGUCY2C cells by tail vein to produce lung metastases, followed 3 days later by TBI and increasing doses of mouse CAR-T cells (Fig. 3A). hGUCY2C-targeted 5F9.m28BBz, but not control, CAR-T cells improved survival of mice at a dose of 107 T cells (Fig. 3A). This dose also was effective when administered 7 days after cancer cell inoculation (Fig. 3B), and a second dose administered on day 14 further increased median survival compared to a single dose on day 7 (>150 vs 93.5 days, p<0.05; Fig. 3C). Lungs collected 18 days after cancer cell inoculation (11 days after treatment) contained tumor nodules, confirming that control mice succumbed to lung metastases while 5F9.m28BBz CAR-T cell treatment eliminated macroscopic tumors (Fig. 3D). To determine if surviving mice exhibited persistent protection against hGUCY2C-expressing tumors, long-term survivors (161-282 days following initial cancer cell inoculation) were challenged with either parental CT26 or CT26.hGUCY2C cells by tail vein injection to examine hGUCY2C-specific protection (Fig. 3E). CT26 tumors are known to harbor the gp70 envelope protein derived from murine leukemia virus that generates protective gp70-specific CD8+ T-cell responses in some vaccination regimens (27). Here, long-term surviving and naïve mice challenged with parental CT26 cancer cells exhibited identical death rates, indicating that long-term survivors did not produce a protective immune response to gp70 or other antigens expressed in CT26 cells (Fig. 3E). Conversely, long-term survivors were protected against CT26.hGUCY2C cancer cells compared to naïve control mice, indicating that 5F9.m28BBz CAR-T cells produce persistent protection against hGUCY2C-expressing tumors (Fig. 3E).
Figure 3. hGUCY2C CAR-T cells provide long-term protection in a syngeneic lung metastasis model.
(A–E) BALB/c mice were injected with 5x105 CT26.hGUCY2C cells via the tail vein to establish lung metastases. Control (4D5.m28BBz) or 5F9.m28BBz CAR constructs were transduced into murine CD8+ T cells. (A) Mice were treated 3 days later with 5 Gy total body irradiation (TBI) followed by 106–107 5F9.m28BBz (N=7–8/group) or 107 control (N=6) CAR-T cells. (B) Mice were treated on day 3 (D3) or day 7 (D7) with 5 Gy TBI followed by 107 control (N=10/group) or 5F9.m28BBz (N=9–10/group) CAR-T cells. (C) Mice were treated on day 7 with 5 Gy TBI followed by 107 control (N=10) or 5F9.m28BBz (N=12) CAR-T cells on day 7 and day 14. (D) Mice treated on day 7 with 5 Gy TBI and PBS or 107 control or 5F9.m28BBz CAR-T cells were sacrificed on day 18, lungs stained with India ink, and tumors/lung enumerated. One-way ANOVA; *p<0.05. (E) Surviving mice from B and C treated with 5F9.m28BBz CAR-T cells or naïve mice were challenged with 5x105 CT26 (N=4–7/group) or CT26.hGUCY2C (N=7/group) cells (re-challenge occurred 16–40 weeks after initial challenge). Log-rank Mantel-Cox test, A–C and E; **p<0.01, ***p<0.001, ****p<0.0001. Up arrows indicate CAR-T cell treatment days. Each panel indicates an independent experiment.
hGUCY2C CAR-T cells recognize human colorectal tumors
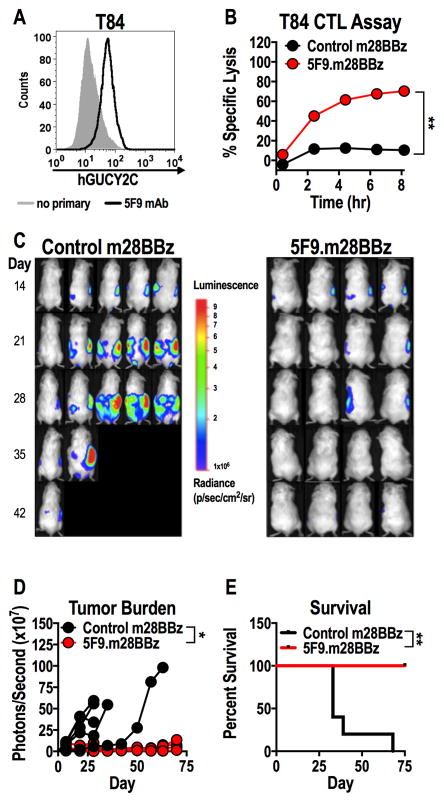
We next determined if hGUCY2C CAR-T cells recognized native hGUCY2C on human colorectal tumors. The recombinant hGUCY2C-specific antibody 5F9 stained hGUCY2C on the surface of GUCY2C-expressing T84 (Fig. 4A), but not GUCY2C-deficient SW480 (Supplementary Fig. S9A), human colorectal cancer cells. Correspondingly, 5F9.m28BBz CAR-T cells lysed T84 (Fig. 4B), but not SW480 (Supplementary Fig. S9B), cancer cells in vitro in a time-dependent manner. Control CAR-T cells did not kill either human cancer cell type, indicating that killing was antigen-dependent (Fig. 4B and Supplementary Fig. S9B). Human T cells expressing a human 5F9 CAR construct (5F9.h28BBz) produced effector cytokines following GUCY2C stimulation and killed human colorectal cancer cells endogenously expressing hGUCY2C (Supplementary Fig. S10). Mouse T cells expressing hGUCY2C-specific (5F9.m28BBz), but not control, CAR effectively treated T84 human colorectal tumor xenografts in a peritoneal metastases model (Fig. 4C–E). Together, these data indicated that hGUCY2C-specific CAR constructs produced with the 5F9 scFv can redirect T cel mediated killing of human colorectal tumors endogenously expressing hGUCY2C.
Figure 4. hGUCY2C CAR-T cells eliminate human colorectal tumor xenografts.
(A) hGUCY2C expression on T84 human colorectal cancer cells was quantified by flow cytometry using the recombinant 5F9 antibody. (B–E) Control (1D3.m28BBz) or 5F9.m28BBz CAR constructs were transduced into murine CD8+ T cells. (B) T84 colorectal cancer cells in an E-Plate were treated in duplicate with 5F9-m28BBz or control CAR-T cells (5:1 E:T ratio), media, or 10% Triton-X 100 (Triton), and the relative electrical impedance was measured every 15 minutes for 20 hours to quantify cancer cell death (normalized to time=0). Percent specific lysis values were calculated using impedance values following the addition of media and Triton for normalization (0% and 100% specific lysis, respectively). Two-way ANOVA; **p<0.01; representative of two independent experiments. (C–E) Immunodeficient NSG mice were injected with 2.5x106 luciferase-expressing T84 colorectal cancer cells via intraperitoneal injection and were treated with 107 control (N=5/group) or 5F9-m28BBz (N=4/group) CAR-T cells on day 14 by intraperitoneal injection. (C–D) Total tumor luminescence (photons/second) was quantified just prior to T-cell injection and weekly thereafter. Two-way ANOVA; *p<0.05. (E) Mice were followed for survival. Log-rank Mantel Cox test; *p<0.05.
Adoptive T-cell therapies targeting colorectal tumor antigens have been limited by antigen “on-target, off-tumor” toxicities (4,5). We previously validated GUCY2C as a potential target for CAR-T cell treatment in a completely syngeneic mouse model in which CARs targeting mouse GUCY2C promoted antitumor efficacy in the absence of toxicities to the normal GUCY2C-expressing intestinal epithelium (12). Here, we produced a human GUCY2C-specific CAR from an antibody that is currently employed as an antibody-drug conjugate in clinical trials for GUCY2C-expressing malignancies (NCT02202759, NCT02202785) and demonstrated its ability to induce T-cell activation, effector function, and antitumor efficacy in both syngeneic and human colorectal tumor xenograft mouse models using murine T cells. CARs produced from the 5F9 scFv do not cross-react with murine GUCY2C (Supplementary Fig. S11), preventing quantification of intestinal toxicity in mouse models. Differences in the antigen-recognition domain of the CAR described here and the murine CAR previously described (12), as well as inherent differences between mice and humans, suggest caution in GUCY2C CAR-T cell administration to humans, despite murine GUCY2C CAR-T cell safety data (12). Thus, appropriate safety measures should be considered when translating the use of GUCY2C CAR-T cells into the clinic, including transient CAR expression by mRNA electroporation (28) or incorporation of suicide genes (29). Nevertheless, GUCY2C-targeted CAR-T cells are an attractive tool for the T-cell therapy armamentarium, a paradigm that is limited by the lack of suitable antigen targets (30). Following further
Supplementary Material
Acknowledgments
Financial support was provided by: NIH (P30 CA56036, R01 CA204881, R01 CA206026 to S. Waldman; F31 CA171672 to M. Magee); Targeted Diagnostic & Therapeutics, Inc. (to S. Waldman); Courtney Ann Diacont Memorial Foundation (to S. Waldman); PhRMA Foundation (to A. Snook); the W.W. Smith Charitable Trust (to A. Snook); and Margaret Q. Landenberger Research Foundation (to A, Snook). S. Waldman is the Samuel M.V. Hamilton Professor of Medicine of Thomas Jefferson University. This project was funded, in part, by grants from the Pennsylvania Department of Health (SAP #4100059197, SAP #4100051723 to S. Waldman). The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing Interests: S. Waldman was the Chair of the Data Safety Monitoring Board for the CHART-1 Trial™ sponsored by Cardio3 Biosciences; and is the Chair of the Scientific Advisory Board and a member of the Board of Directors (both uncompensated) of Targeted Diagnostics & Therapeutics, Inc. which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work.
References
- 1.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustaind remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19(3):620–6. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–51. doi: 10.1038/mt.2010.24. mt201024 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aka AA, Rappaport JA, Pattison AM, Sato T, Snook AE, Waldman SA. Guanylate cyclase C as a target for prevention, detection, and therapy in colorectal cancer. Expert review of clinical pharmacology. 2017;10(5):549–57. doi: 10.1080/17512433.2017.1292124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valentino MA, Lin JE, Snook AE, Li P, Kim GW, Marszalowicz G, et al. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest. 2011;121(9):3578–88. doi: 10.1172/JCI57925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrithers SL, Barber MT, Biswas S, Parkinson SJ, Park PK, Goldstein SD, et al. Guanylyl cyclase C is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc Natl Acad Sci U S A. 1996;93(25):14827–32. doi: 10.1073/pnas.93.25.14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birbe R, Palazzo JP, Walters R, Weinberg D, Schulz S, Waldman SA. Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum Pathol. 2005;36(2):170–9. doi: 10.1016/j.humpath.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe HR, Mendizabal M, Lleong E, Cuthbertson A, Desai V, Pullan S, et al. In vivo imaging of human colon cancer xenografts in immunodeficient mice using a guanylyl cyclase C--specific ligand. J Nucl Med. 2002;43(3):392–9. [PubMed] [Google Scholar]
- 11.Coradini D, Casarsa C, Oriana S. Epithelial cell polarity and tumorigenesis: new perspectives for cancer detection and treatment. Acta Pharmacol Sin. 2011;32(5):552–64. doi: 10.1038/aps.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magee MS, Kraft CL, Abraham TS, Baybutt TR, Marszalowicz GP, Li P, et al. GUCY2C-directed CAR-T cells oppose colorectal cancer metastases without autoimmunity. Oncoimmunology. 2016;5(10):e1227897. doi: 10.1080/2162402x.2016.1227897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marszalowicz GP, Snook AE, Magee MS, Merlino D, Berman-Booty LD, Waldman SA. GUCY2C lysosomotropic endocytosis delivers immunotoxin therapy to metastatic colorectal cancer. Oncotarget. 2014;5(19):9460–71. doi: 10.18632/oncotarget.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snook AE, Li P, Stafford BJ, Faul EJ, Huang L, Birbe RC, et al. Lineage-specific T-cell responses to cancer mucosa antigen oppose systemic metastases without mucosal inflammatory disease. Cancer Res. 2009;69(8):3537–44. doi: 10.1158/0008-5472.CAN-08-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snook AE, Magee MS, Schulz S, Waldman SA. Selective antigen-specific CD4(+) T-cell, but not CD8(+) T- or B-cell, tolerance corrupts cancer immunotherapy. Eur J Immunol. 2014;44(7):1956–66. doi: 10.1002/eji.201444539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodson CA, Ambrogi IG, Scott RO, Mohler PJ, Milgram SL. Polarized apical sorting of guanylyl cyclase C is specified by a cytosolic signal. Traffic. 2006;7(4):456–64. doi: 10.1111/j.1600-0854.2006.00398.x. TRA398 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Charney AN, Egnor RW, Alexander-Chacko JT, Zaharia V, Mann EA, Giannella RA. Effect of E. coli heat-stable enterotoxin on colonic transport in guanylyl cyclase C receptor-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;280(2):G216–21. doi: 10.1152/ajpgi.2001.280.2.G216. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn M, Adermann K, Jahne J, Forssmann WG, Rechkemmer G. Segmental differences in the effects of guanylin and Escherichia coli heat-stable enterotoxin on Cl- secretion in human gut. The Journal of Physiology. 1994;479(Pt 3):433–40. doi: 10.1113/jphysiol.1994.sp020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guarino A, Cohen MB, Overmann G, Thompson MR, Giannella RA. Binding of E. coli heat-stable enterotoxin to rat intestinal brush borders and to basolateral membranes. Dig Dis Sci. 1987;32(9):1017–26. doi: 10.1007/BF01297193. [DOI] [PubMed] [Google Scholar]
- 20.Witek ME, Nielsen K, Walters R, Hyslop T, Palazzo J, Schulz S, et al. The putative tumor suppressor Cdx2 is overexpressed by human colorectal adenocarcinomas. Clin Cancer Res. 2005;11(24 Pt 1):8549–56. doi: 10.1158/1078-0432.CCR-05-1624. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, et al. Affinity-Tuned ErbB2 or EGFR Chimeric Antigen Receptor T Cells Exhibit an Increased Therapeutic Index against Tumors in Mice. Cancer Res. 2015;75(17):3596–607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mule JJ, Shu S, Schwarz SL, Rosenberg SA. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984;225(4669):1487–9. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- 23.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 24.Xu XJ, Song DG, Poussin M, Ye Q, Sharma P, Rodriguez-Garcia A, et al. Multiparameter comparative analysis reveals differential impacts of various cytokines on CART cell phenotype and function ex vivo and in vivo. Oncotarget. 2016 doi: 10.18632/oncotarget.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, et al. Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go? Nat Clin Pract Oncol. 2006;3(12):668–81. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang AY, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, et al. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci U S A. 1996;93(18):9730–5. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2(2):112–20. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casucci M, Bondanza A. Suicide gene therapy to increase the safety of chimeric antigen receptor-redirected T lymphocytes. J Cancer. 2011;2:378–82. doi: 10.7150/jca.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg SA. Decade in review-cancer immunotherapy: Entering the mainstream of cancer treatment. Nature reviews Clinical oncology. 2014 doi: 10.1038/nrclinonc.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.