Abstract
A number of studies have reported comorbidity of food allergies with various neuropsychiatric disorders, such as anxiety, depression, attention-deficit hyperactivity disorder, and autism. However, inconsistent results across clinical studies have left the association between food allergy and behavioral disorders inconclusive. We postulated that the heterogeneities in genetic background among allergic cohorts affect symptom presentation and severity of food allergy, introducing bias in patient selection criteria toward individuals with overt physical reactions. To understand the influence of genetic background on food allergy symptoms and behavioral changes beyond anaphylaxis, we generated mouse models with mild cow’s milk allergy by sensitizing male and female C57BL/6J and BALB/cJ mice to a bovine whey protein, β-lactoglobulin (BLG; Bos d 5). We compared strain- and sex-dependent differences in their immediate physical reactions to BLG challenge as well as anxiety-like behavior one day after the challenge. While reactions to the allergen challenge were either absent or mild for all groups, a greater number of BLG-sensitized BALB/cJ mice presented visible symptoms and hypothermia compared to C57BL/6J mice. Interestingly, male mice of both strains displayed anxiety-like behavior on an elevated zero maze without exhibiting cognitive impairment with the cross maze test. Further characterization of plasma cytokines/chemokines and fecal microbiota also differentiated strain- and sex-dependent effects of BLG sensitization on immune-mediator levels and bacterial populations, respectively. These results demonstrated that the genetic variables in mouse models of milk allergy influenced immediate physical reactions to the allergen, manifestation of anxiety-like behavior, levels of immune responses, and population shift in gut microbiota. Thus, stratification of allergic cohorts by their symptom presentations and severity may strengthen the link between food allergy and behavioral disorders and identify a population(s) with specific genetic background that have increased susceptibility to allergy-associated behavioral disorders.
Keywords: Cow’s milk allergy, anxiety, behavior, cytokine, chemokine, growth factor, microbiota, dysbiosis, Akkermansia
1. INTRODUCTION
Food allergy, defined as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food” (Boyce et al., 2010), is an increasingly prevalent health concern worldwide (Mullins, 2007; Liu et al., 2010; Gupta et al., 2018; Loh and Tang, 2018; Sicherer and Sampson, 2018). In the United States, where approximately 8–10% of children and adults are afflicted with food allergy, cow’s milk allergy (CMA) has been reported as the second most common food allergy in both age groups (Gupta et al., 2018; Gupta et al., 2019). Clinical presentations of CMA can vary across individuals, and their manifestations may be immediate or delayed (Hill and Hosking, 1995; Baehler et al., 1996; Koletzko et al., 2012; Dupont, 2014). Symptoms that are typically recognized as “allergic reactions” include edema, hives, diarrhea, vomiting, respiratory distress, and systemic anaphylaxis, which occur immediately after ingestion of milk via immunoglobulin E (IgE)-mediated responses (du Toit et al., 2010; Burks et al., 2012; Mousan and Kamat, 2016). Delayed symptoms of CMA are more generalized cutaneous and gastrointestinal discomfort, such as eczema and constipation, and can emerge several hours to days following milk consumption via IgE-independent mechanisms (du Toit et al., 2010; Mousan and Kamat, 2016).
In addition to the clinical presentations mentioned above, mood, cognitive, and behavioral symptoms have been associated with CMA, and thus, neuropsychiatric disorders such as anxiety, depression, attention deficit hyperactivity disorder (ADHD), and autism may partly be neurological manifestations of hypersensitivity to cow’s milk proteins and other dietary allergens in some individuals (Davison, 1949; Speer, 1954; Boris and Mandel, 1994; Hak et al., 2013; Lyall et al., 2015; Topal et al., 2016; Xu et al., 2018). In support of this notion, removal of suspected food from patients’ diet has been reported to alleviate their symptoms while reintroduction exacerbates them (Davison, 1949; Speer, 1954; Boris and Mandel, 1994; Stevens et al., 2010). Furthermore, oral immunotherapy of children with CMA was found to significantly improve anxiety (Carraro et al., 2012). Despite a large number of case reports and cohort studies that demonstrated positive correlations between neuropsychiatric conditions and atopic diseases (Afari et al., 2001; Heaney et al., 2005; Mostafa et al., 2008; Yaghmaie et al., 2013; Garg and Silverberg, 2014; Lyall et al., 2015; Ferro et al., 2016; Goodwin et al., 2017; Busquets et al., 2019; Blondal et al., 2020), CMA or other food allergies as a pathophysiological trigger of mood and behavioral symptoms has not been fully acknowledged in the field, but rather perceived as a psychological trigger of fear arising from the anticipation for accidental exposures to offending allergens (Cummings et al., 2010; Walkner et al., 2015; Herbert et al., 2016; Polloni and Muraro, 2020). Inconsistent results across clinical studies, perhaps due to multiple variables associated with the cohorts, have also likely contributed as inconclusive evidence for the role of food allergy as a causal factor for neuropsychiatric symptoms. Indeed, variables, such as genetic background and ethnicity, diet, and medical history, are challenging to normalize with human subjects in addition to the differences in the presentations, number, and severity of food allergies. Furthermore, intestinal microbiota, which has been increasingly implicated in both allergy (Inoue et al., 2017; Kourosh et al., 2018; Hussain et al., 2019) and neuropsychiatric disorders (Finegold et al., 2002; Wang et al., 2013; Naseribafrouei et al., 2014; Kelly et al., 2016; Gupta et al., 2019), is another possible variable that may affect study outcomes and should be taken into consideration.
Mouse models, therefore, provide valuable tools by allowing researchers to control many of these variables. Previously, we and others demonstrated that allergic sensitization to cow’s milk proteins elicited behavioral abnormalities in otherwise healthy wild-type mice (de Theije et al., 2014; Germundson et al., 2018; Smith et al., 2019; Germundson et al., 2020). Moreover, these studies showed that the increases in c-Fos immunoreactivity (de Theije et al., 2014), degranulated mast cell numbers (Germundson et al., 2018), astrogliosis (Germundson et al., 2018; Smith et al., 2019), and proinflammatory cytokines (Smith et al., 2019) were found in the brains of allergen-sensitized mice. These results suggested that behavioral symptoms in food allergy were more than comorbidity, prompting further investigation of CMA as a causative factor that could influence brain function and behavior.
In our mouse model, CMA-associated behavioral changes were observed only in male mice and not in female mice (Germundson et al., 2018; Smith et al., 2019). Interestingly, sex differences have also been reported in humans for behavioral disorders and food allergies (Altemus, 2006; Kelly and Gangur, 2009; Acker et al., 2017; Xu et al., 2018; Murray et al., 2019). Together with individual differences in offending allergen types, symptom presentations, and reaction severity, the susceptibility toward CMA-associated behavioral manifestations may depend on the genetic background of allergic individuals.
In this study, we therefore examined the influence of strain and sex differences in CMA-induced behavioral manifestations using male and female mice of two genetically distinct strains, C57BL/6J and BALB/cJ. Mice were sensitized to β-lactoglobulin (BLG: Bos d 5), a major allergen in bovine whey, for 5 weeks and challenged with BLG during the 6th week to assess their immediate physical reactions. Anxiety-like behavior and spatial memory were also tested one day after the allergen challenge. In addition, fecal microbiomes were compared among the mouse groups to determine the potential contribution of sex and strain in influencing intestinal microbiota after BLG sensitization. Our results indicated that these genetic variables significantly affected CMA sequelae and their extent, including immediate reactions to the allergen, behavior changes, systemic cytokine levels, and intestinal microbiota.
2. MATERIALS AND METHODS
2.1. Reagents and antibodies
Purified BLG (#L0130) was purchased from MilliporeSigma (Burlington, MA, U.S.A.) and used as the allergen for sensitization. Azide-free cholera toxin (CT: #100B) from Vibrio cholerae was obtained from List Biological Laboratories (Campbell, CA, U.S.A.) and used as the adjuvant. For isotype-specific immunoglobulin ELISA, Dynabeads™ Protein G, anti-mouse IgE, IgG1, and IgG2a secondary antibodies, and ELISA Support Pack Plus were purchased from ThermoFisher Scientific (Waltham, MA, U.S.A.). The secondary antibodies were used at 1:1,000. The ELISA Support Pack Plus contained TMB (3,3’,5,5’-tetramethylbenzidine) and phosphoric acid as the substrate and stop solution, respectively. For quantitative cytokine arrays, the slide-based Quantibody® Mouse Cytokine Array Q5 (QAM-CYT-5) was acquired from RayBiotech Life (Peachtree Corners, GA, U.S.A.). The fluorescence signals from the array experiments were quantified by the manufacturer.
2.2. Mice
Three-week-old male and female C57BL/6J and BALB/cJ mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Upon arrival, mice were caged by sex and randomly divided into groups of 5 mice per cage before housed under a 12-h light/12-h dark cycle in a specific-pathogen-free environment at the University of North Dakota animal facility. Ten mice were used per experimental group. All animals were given ad libitum access to a whey-free rodent diet (Teklad 2018, Envigo, Indianapolis, IN) and ultra-filtered water. Body weights of mice were recorded weekly to assess health and growth throughout the duration of the experiment. All procedures involving mice were approved by the University of North Dakota Institutional Animal Care and Use Committee prior to experiments.
2.3. Stool sample collection
After 1 week of acclimation with the whey-free diet, each mouse was individually placed in a cage until stool samples were collected and returned to its home cage with original cage mates. Fecal pellets from each mouse were collected in an autoclaved microfuge tube using sterile forceps and placed on ice until pellets were harvested from all mice. Stool samples were frozen stored at −80 °C until used for microbial DNA extraction. Additional fecal samples were collected in the same manner immediately prior to euthanasia to assess post-sensitization changes in fecal microbiomes.
2.4. Allergen sensitization and challenge
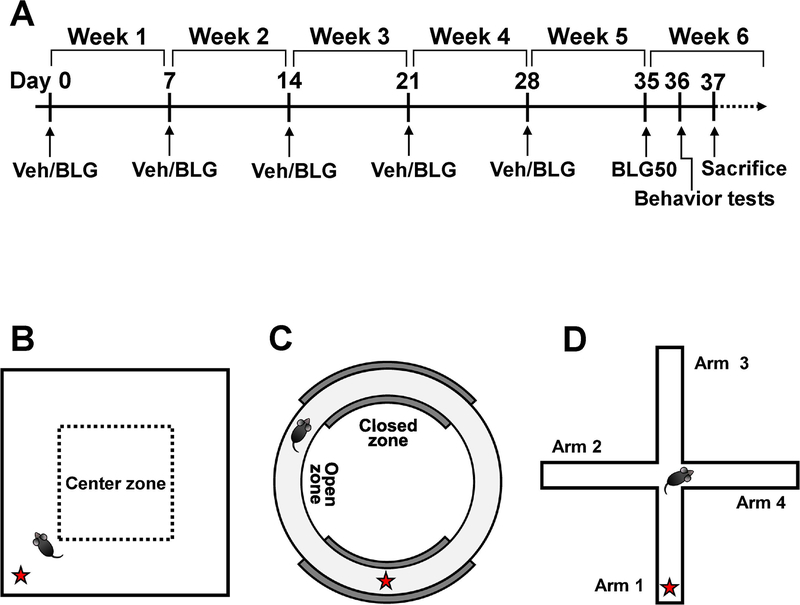
The sensitization procedure was carried out based on the protocol by (Li et al., 1999; Berin et al., 2006) as described previously (Smith et al., 2019; Smith and Nagamoto-Combs, 2020). Briefly, sex- and strain-matched 4-week-old mice were randomly assigned to sham and BLG-sensitization groups and given weekly intragastric gavage for 5 weeks (Fig. 1A). For each gavage, sham and BLG mice were fasted for 2 hours prior and received 200 μL of either vehicle or BLG solution, respectively. The vehicle contained 10 μg CT as an adjuvant in 200 μL sodium carbonate/bicarbonate buffer (0.2 M, pH 9.0), while the BLG solution contained 1 mg BLG in the vehicle. Mice were returned to their home cages without food for additional 2 hours before allowing access to the whey-free diet. At Week 6, all mice were fasted for 2 hours and challenged with 50 mg BLG in 200 μL sodium carbonate/bicarbonate buffer (0.2 M, pH 9.0). Physical symptoms were scored at 30-min post-challenge according to the anaphylaxis scoring scale described by Li et al. (1999)(Li et al., 1999) (Table 1). To assess the degree of challenge-induced hypothermia, body temperature was recorded using a MicroTherma 2T Handheld Thermometer with a RET-3 probe (Braintree Scientific, Inc., Braintree, MA, U.S.A.). Mice were returned to their home cages and allowed to rest for one day until behavior tests.
Figure 1. Experimental timeline and behavior tests.
(A) Starting at 4-weeks of age, mice were administered 200 μL of vehicle containing 10 μg cholera toxin only (Veh) or the vehicle with 1 mg BLG (BLG) by gavage weekly from Week 1 through Week 5. During week 6, all mice were challenged with 50 mg BLG in sodium carbonate/bicarbonate buffer (BLG50). One day after the challenge, open field activity monitoring (B) elevated zero maze (C), and cross maze (D) tests were performed to assess behavior. The red star in each diagram indicates the starting location.
Table 1. Anaphylaxis scoring scales.
Scores and symptom descriptions adapted from Li et al., 1999.
| 0 | No reaction/clinical symptoms |
| 1 | Scratching and rubbing around the nose and head |
| 2 | Puffiness around the eyes and mouth, pilar erecti, reduced activity |
| 3 | Wheezing, labored respiration and cyanosis around the mouth and tail |
| 4 | No activity after prodding or tremor and convulsion |
| 5 | Death |
2.5. Behavioral assessments
2.5.1. Open-field (OF) activity monitoring
A plexiglass enclosure (40.6-cm width × 40.6-cm depth × 38.1-cm height; San Diego Instruments, San Diego, CA, U.S.A.) with a defined 20-cm × 20-cm center zone was used (Fig. 1B). Mice were individually placed in the same area of the enclosure outside of the center zone (marked with a red star in Fig. 1B) and allowed to freely explore for 10 min, and their spontaneous activities were recorded by an overhead digital video camera. The number of entries into the center zone of the box, total time spent in the center zone, the average duration of visit to the center zone, and the number of fecal pellets produced during the test duration, average speed, distance traveled, and time immobile were recorded to objectively assess anxiety-like behavior as well as overall activity levels using ANY-maze software (Stoelting Co., Wood Dale, IL, U.S.A.). The number of fecal pellets was manually counted from the video recordings by two blinded observers as a mobility-independent measure of anxiety-like behavior. The enclosure was thoroughly cleaned with Process NPD (STERIS, Mentor, OH, U.S.A.) between tests.
2.5.2. Elevated zero maze (EZM)
A commercially available EZM apparatus (Stoelting Co.) was used to measure anxiety-like behavior as previously described (Smith et al., 2019). Briefly, mice were individually placed in one of the walled sections of the circular maze (Fig. 1C) and allowed to freely explore the apparatus for 10 min while being video recorded. The time spent in the open zones, the number of entries into the open zones, and the average duration of visit to the open zones were analyzed using ANY-maze software and manually validated by a blinded observer. A few mice failed to stay on the maze for the entire test duration, and they were excluded from the final analysis.
2.5.3. Cross maze
A cross maze was used to evaluate spatial working memory. The maze was custom-made with white plexiglass with four identical cross-shaped arms (5-cm width × 30-cm length) with a 5-cm × 5-cm center area and 15-cm high walls based on the specifications by Kulas et al. (2018) (Kulas et al., 2018). Mice were individually placed in one of the arms designated as the starting arm and allowed to explore the maze freely for 12 min (Fig. 1D). The sequence of arm entries was recorded by an overhead digital video camera and analyzed by a blinded observer. “Alternations” was defined as the instances where the mouse successfully entered all 4 arms of the maze without reentering a previously entered arm. The percentage of alternations was calculated as using the following equation (Kulas et al., 2018):
2.6. Euthanasia and terminal blood sample collection
Mice were sacrificed 2 days after the allergen challenge and 1 day after the behavioral assessments (Fig. 1A). After post-sensitization stool collection, mice were euthanized via CO2 asphyxiation followed by intracardiac perfusion with phosphate-buffered saline (PBS; pH 7.4). Cardiac blood was collected prior to perfusion to prepare serum and plasma samples for immunoglobulin and cytokine ELISAs, respectively. For plasma samples, approximately 100 μL of blood was collected in EDTA-coated Microvette tubes (Sarstedt, Inc., Newton, NC, U.S.A.) and centrifuged for 10 min at 2,000 × g at 4 °C. For serum preparation, blood was collected in microfuge tubes and allowed to coagulate for 30 min at room temperature before centrifuged at 2,000 × g for 15 min. Aliquoted serum and plasma samples were stored at −80 °C until used.
2.7. Enzyme-linked immunosorbent assay (ELISA) for BLG-specific immunoglobulin detection
BLG-specific immunoglobulins were detected using ELISA as previously described (Smith et al., 2019; Germundson and Nagamoto-Combs, 2020). Briefly, 96-well ELISA plates were coated with 20 μg/mL BLG in 100 mM sodium carbonate/bicarbonate buffer (pH 9.6) overnight at 4 °C, rinsed with PBS containing 0.05% Tween-20 (PBS-T) and blocked with PBS-T with 0.5% bovine serum albumin. Serum samples from mice were first diluted to 1:40 to adsorb IgG using magnetic protein G beads, and resulting supernatants were used for BLG-specific IgE detection. Bead-bound IgG was eluted in 50 mM glycine (pH 2.8) for 2 min at room temperature, neutralized with 1 M Tris buffer (pH 7.5), and used to detect BLG-specific IgG1 and IgG2a. Samples were placed in the allergen-coated wells and incubated overnight at 4 °C. BLG-specific immunoglobulins were detected by respective anti-mouse secondary antibodies followed by avidin-HRP and using TMB as the substrate. Reactions were terminated by adding 2 N H2SO4 prior to reading at 450 nm on a Biotek ELx 800 microplate reader with Gen5 software (Biotek Instruments).
2.8. Cytokine quantification
Plasma samples prepared from terminal blood were used to profile cytokines and chemokines using Quantibody® Mouse Cytokine Array Q5 Kit. Samples were first diluted to 1:5 before assayed using the multiplex kit according to the manufacturer’s instructions. Upon completion of the assay procedure, the slides were sent to RayBiotech Array Scanning and Analysis Services for quantification of the fluorescence signals. The resulting fluorescence signal values were analyzed using RayBiotech Q-Analyzer® an array-specific Microsoft Excel-based software tool (https://www.raybiotech.com/files/analysis-tools/QAM-CYT-5.xls).
2.9. Fecal microbiome analysis
To compare fecal microbiome profiles, microbial DNA was extracted from stool samples using ZymoBIOMICS DNA kit (Zymo Research, Irvine, CA, U.S.A.) and sent to the Genome Technology Access Center at Washington University (St. Louis, MO, U.S.A.) for 16S ribosomal RNA gene sequencing. Sample library preparation for 8 hypervariable regions of the 16S gene was performed with Fluidigm Juno LP 192.24 IFC system. Sequencing was carried out on a HiSeq 3000 with approximately 11M 150 bp paired-end reads, yielding an average of 44,277 reads per sample. The raw FASTQ files were assessed for quality by DADA2. The forward and reverse reads were truncated using filterAndTrim function of DADA2 package at 140 and 130 base position, respectively. After dereplication by DADA2, filtered paired-end reads were merged, and a quality-aware correction model was used to remove noise and chimeras to call final amplicon sequence variants (ASVs) (Callahan et al., 2017). ASVs were classified taxonomically based on the SILVA database (version 132, https://zenodo.org/record/1172783#.X3zfvy2ZOL8), and the ASVs that were not assigned to phyla or assigned to non-bacterial kingdoms by the phyloseq R package (version 1.32.0) (McMurdie and Holmes, 2013) were removed. Furthermore, the ASVs with prevalence < 9 and total abundance < 222 (lower quartile) were excluded from downstream analysis. Taxonomy classification for trimmed reads was performed by Kraken 2 (version 2.0.8-beta) (Wood et al., 2019) based on MiniKraken2_v1 database (version 201904). Differential species were screened out by the DESeq2 package (version 1.28.1).
The alpha diversity metrics were analyzed using the estimate_richness function. Proportionally normalized data was used for Bray-Curtis Principal Coordinates Analysis (PCoA) to reveal differences between experimental groups. The ‘Adonis’ feature from the vegan (version 2.5–6) R package was used to assess whether sample grouping by metadata factor accounted for inter-sample differences (Oksaren et al., 2020). The DESeq2 package (version 1.28.1) was used to identify significant differences in ASVs between groups (Love et al., 2014). Differential ASVs were then subjected to KEGG pathway profile analysis using Tax4Fun2 R package (version 1.1.5) (Wemheuer et al., 2020) with ‘Ref99NR’ database mode and Tax4Fun2_ReferenceData_v1.1. KEGG pathways with adjusted p value < 0.05 were considered significant after multiple testing correction.
2.10. Species-specific quantitation of Akkermansia muciniphila
Abundance of A. muciniphila at pre- and post-sensitization was detected from 100 ng of microbial DNA using quantitative PCR (qPCR). The species-specific primers, S-St-Muc-1129-a-a-20: 5’-CAG CAC GTG AAG GTG GGG AC-3’ and S-St-Muc-1437-a-A-20: 5’ - CCT TGC GGT TGG CTT CAG AT-3’ (Collado et al., 2007) and iTaq™ Universal SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA, U.S.A) were used to perform qPCR on a C1000 Touch Thermocycler (Bio-Rad) for 40 cycles (denaturing at 95 °C for 15 sec, annealing at 55 °C for 30 sec, and extension at 72 °C for 30 sec). Cq values were computed using Bio-Rad CFX Manager 3.1, and the amount of A. muciniphila was expressed as 2−Cq.
2.11. Statistics
All statistical analyses, except the microbiome analysis, were performed using GraphPad Prism 9 software (GraphPad Software, Inc., San Diego, CA, U.S.A.). Differences between strain-matched sham and BLG groups and between treatment-matched two strains were compared independently among male and female groups using two-way ANOVA with Fisher’s least significant difference (LSD) test, unless specified in the figure legends. The ROUT method (Q=1%) was used to identify outliers in a group when appropriate, and the values were removed from the final results. A p value less than 0.05 (p < 0.05) was considered statistically significant.
3. RESULTS
3.1. BLG sensitization produced distinct physical responses in C57BL/6J and BALB/cJ mouse strains upon BLG challenge.
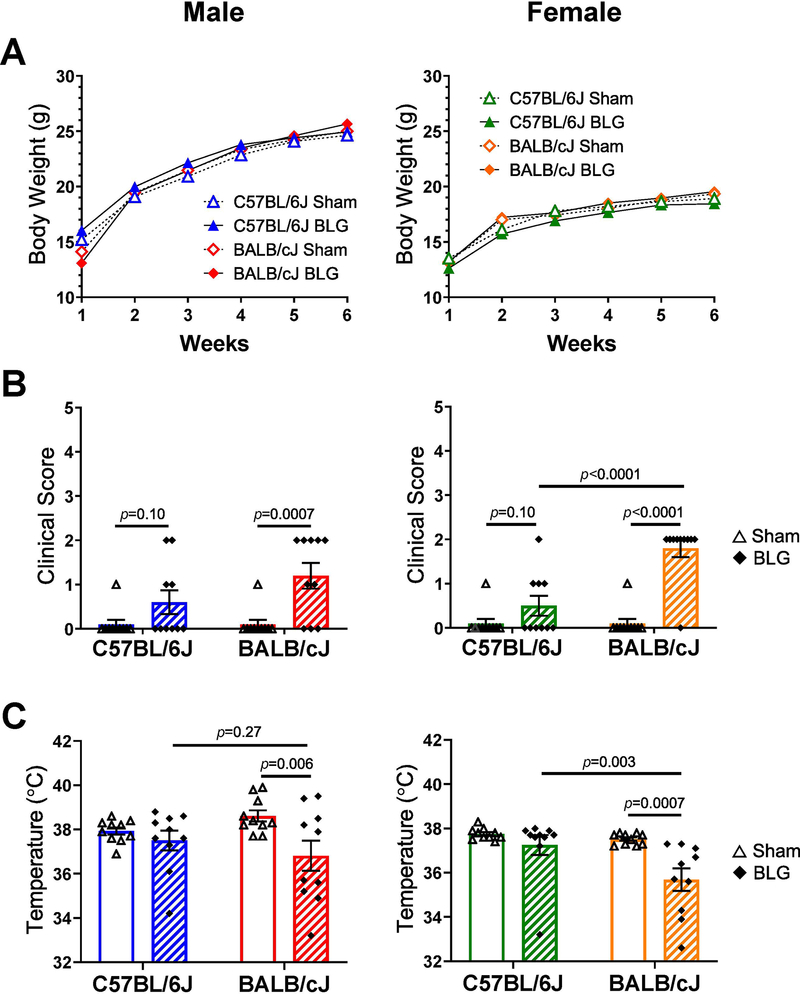
During the 6 weeks of sensitization, body weights of mice were recorded weekly to monitor overall health. As observed in our previous study (Smith et al., 2019), BLG sensitization had no impact on the overall growth of sex-matched mice (Fig. 2A). When challenged with 50 mg of BLG in Week 6, most of the C57BL/6J mice, regardless of sex, were asymptomatic (Fig. 2B) and scored 0 on the anaphylaxis scale (Table 1). A few mice in the BLG-sensitized group showed minor symptoms although the maximum score did not exceed 2, and the differences between the average scores of sex-matched sham and BLG-sensitized groups were not statistically significant (male C57BL/6J sham: 0.10 ± 0.09, male C57BL/6J BLG: 0.6 ± 0.3, p = 0.10; female C57BL/6J sham: 0.10 ± 0.09, female C57BL/6J BLG 0.5 ± 0.2, p = 0.10; n = 10 in all groups). While the sensitized BALB/cJ mice of both sexes also did not score more than 2, many more animals presented with observable symptoms than sex-matched C57BL/6J mice, and the differences between the average scores of the sex-matched sham and BLG groups for BALB/cJ were significantly different (male sham: 0.10 ± 0.09, male BLG: 1.2 ± 0.3, p = 0.0007; female sham: 0.10 ± 0.09, female BLG: 1.8 ± 0.2,p < 0.0001; n = 10 in all groups). Sex- and treatment-matched strain differences in clinical scores were only significant between BLG-sensitized female sham and BLG mice (p < 0.0001). Similarly, when body temperatures were measured to assess allergen-induced hypothermia (Fig. 2C), the majority of BLG-sensitized male and female C57BL/6J mice maintained their normal body temperature at 30 min post-challenge, showing no significant differences between sex-matched sham and BLG groups (male C57BL/6J sham: 37.9 ± 0.2; male C57BL/6J BLG: 37.5 ± 0.4; female C57BL/6J sham: 37.75 ± 0.09; female C57BL/6J BLG: 37.3 ± 0.4; n = 10 in all groups). However, BLG-sensitized BALB/cJ groups of both sexes clearly presented allergen-induced hypothermia reflective of histaminergic action from mast cells (Makabe-Kobayashi et al., 2002) (male BALB/cJ sham: 38.6 ± 0.3, male BALB/cJ BLG: 36.8 ± 0.7, p = 0.006; female BALB/cJ sham: 37.52 ± 0.06; female BALB/cJ BLG: 35.7 ± 0.5, p = 0.0007; n = 10 in all groups). In addition, as with the clinical scores, BLG-sensitized female BALB/cJ group displayed greater hypothermic responses than sex-matched C57BL/6J mice (p = 0.003). In contrast, the strain differences in allergen-induced hypothermia in BLG sensitized mice were not significant in males (p = 0.27). These results indicated that the severity of allergic responses was sex- and strain-dependent, with female BALB/cJ mice exhibiting most robust reactions among the BLG-sensitized groups tested, followed by male BALB/cJ mice. In contrast, BLG-sensitized C57BL/6J mice displayed minimally observable physical responses upon allergen challenge as we had previously reported (Smith et al., 2019).
Figure 2. Physical responses of mice to BLG sensitization and challenge.
(A) Body weight charts depicting comparable growth of male and female sham (open symbols) and BLG-sensitized (filled symbols) C57BL/6J (triangles) and BALB/cJ (diamonds) mice measured weekly during the sensitization procedure. (B) Clinical symptoms observed by individual mice were determined at 30 min after BLG allergen challenge in Week 6. Symptoms were scored based on the symptom score table (Table 1). (C) Body temperature (°C) measured at 30 min after the allergen challenge. Male C57BL/6J (blue), male BALB/cJ (red); female C57BL/6J (green), female BALB/cJ (orange). Bars in B and C indicate group average values ± SEM (n = 10 per group), two-way ANOVA.
3.2. Allergen-specific immunoglobulins were differentially produced in BLG-sensitized mice in a sex- and strain-dependent manner.
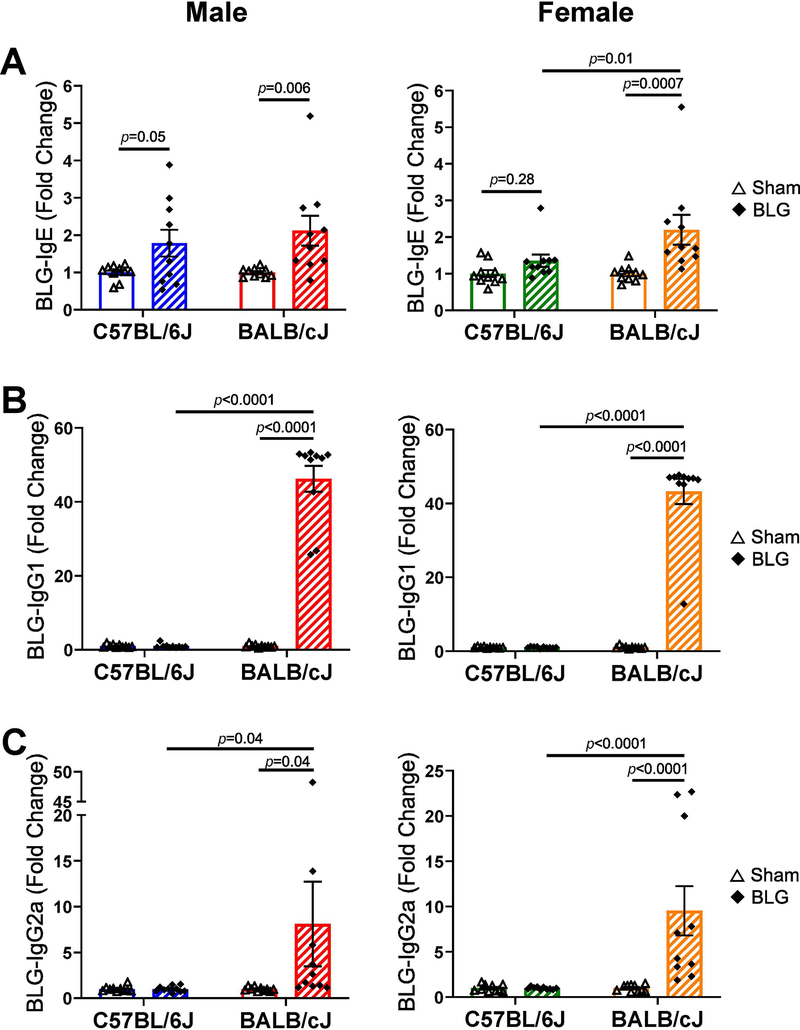
To validate the development of adaptive immunity after the sensitization procedure, subclasses of BLG-specific immunoglobulins, IgE, IgG1, and IgG2a, were individually detected using ELISA (Fig. 3, also see Supplementary Material 1). When compared to respective sham mice, BLG-specific IgE (Fig. 3A) was significantly elevated approximately by 2-fold in the sera of sensitized male C57BL/6J mice (1.8 ± 0.4 fold, n = 10, p = 0.05) and male and female BALB/cJ mice (male: 2.1 ± 0.4 fold, n = 10, p = 0.006; female: 2.20 ± 0.41 fold, n = 10, p = 0.0007), but not in BLG-sensitized female C57BL/6J mice (1.0 ± 0.2 fold, n=10, p = 0.28). In contrast, neither male nor female C57BL/6J mice showed allergen-specific IgG1 production (Fig. 3B) even though robust increases in serum levels of BLG-specific IgG1 was detected in the sensitized BALB/cJ mice of both sexes (male: 46 ± 3 fold, n = 10, p < 0.0001; female BALB/cJ BLG: 43 ± 3 fold, n = 10, p < 0.0001). Similarly, elevated levels of BLG-specific IgG2a were found in both male and female BALB/cJ mice (male: 8 ± 5 fold, n =10, p = 0.04; female: 10 ± 3 fold, n = 10, p < 0.0001) but not in C57BL/6J mice (Fig. 3C). These results indicated that BALB/cJ mice responded to BLG sensitization by productions of all immunoglobulin subclasses tested. In contrast, such response was limited to allergen-specific IgE in male C57BL/6J mice whereas females of this strain did not show significant immune responses after the 5-week sensitization regimen and an allergen challenge. Thus, the sex and strain of mice influenced the extent and the class of allergen-specific immunoglobulins produced after BLG sensitization.
Figure 3. Serum levels of BLG-specific immunoglobulin isotypes.
Terminal blood samples were used to detect BLG-specific serum IgE (A), IgG1 (B) and IgG2a (C) using ELISA. Fold change was calculated by normalizing optical density (OD) values obtained for BLG-sensitized groups to those for sex- and strain-matched sham groups. Sham mice (open bars with open triangles); BLG mice (striped bars with filled diamonds); male C57BL/6J (blue); male BALB/cJ (red); female C57BL/6J (green); female BALB/cJ (orange). Bars indicate group average values in fold changes ± SEM (n = 10 per group), two-way ANOVA.
3.3. Anxiety-like behavior differentially manifested in BLG-sensitized male C57BL/6J and BALB/cJ mice after allergen challenge without affecting general activity and cognitive function.
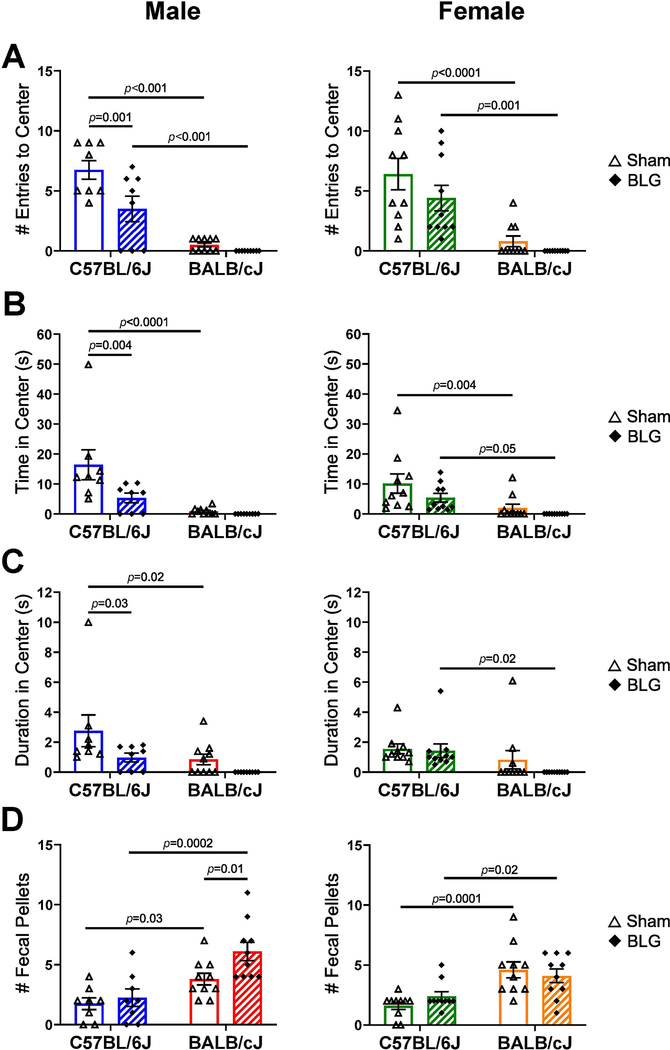
For the OF test, we first tracked the frequency of visits to the center zone, the total time spent in the center zone, and the average duration of each visit to the center zone at 1-min intervals to detect possible time-dependent changes in anxiety-like behavior during the course of the 10-min testing period (Supplementary Material 2). From this preliminary analysis, we observed that significant differences between the sham and BLG groups in the measured parameters were often apparent during the first 4 min as previously reported by others (Bailey et al., 2007; Tanda et al., 2009; Maeta et al., 2018) but became less evident during the latter half of the test duration. Thus, the activity parameters during the first 4 min of the test period were compared among the groups (Fig. 4). BLG-sensitized male C57BL/6J mice made significantly fewer entries to the center zone of the OF arena (sham: 6.8 ± 0.8, n = 8; BLG: 4 ± 1, n = 8, p = 0.001), spent less total time in the center zone (sham: 16 ± 5 sec, n = 8; BLG: 5 ± 2 sec, n = 8, p = 0.004), and spent less time in the center zone per entry (sham: 3 ± 1 sec, n = 8; male C57BL/6J BLG: 1.0 ± 0.3 sec, n = 8, p = 0.03) than their sham counterparts (Fig. 4A–C). The CMA-associated effects on these parameters were not statistically significant for male BALB/cJ mice and female mice of both strains. However, in comparison to C57BL/6J mice, all groups of BALB/cJ mice noticeably avoided the center zone during the test period, posing a challenge in detecting any changes with BLG sensitization. In an attempt to detect differences in anxiety-like behavior between the treatment groups for BALB/cJ mice using another approach, the numbers of fecal pellets produced during the test were counted (Crumeyrolle-Arias et al., 2014; Seibenhener and Wooten, 2015). Greater numbers of fecal pellets, considered to reflect an increased anxiety-like state in rodents, were produced by BLG-sensitized male BALB/cJ mice than their sham control mice (Fig. 4D, sham: 3.8 ± 0.5, n = 10; BLG: 6.1 ± 0.8, n = 10, p = 0.01) although no differences were found in any other groups.
Figure 4. Open-field activity monitoring test.
The overall activity of mice in an open field arena was monitored one day after the BLG challenge. The first 4 min of the test were analyzed to assess differences in the number of entries to the center area (A), total time spent in the center (B), and the duration of each entry to the center (C). In addition, the numbers of fecal pellets excreted during the test were counted as an alternative measure of anxiety-like behavior (D). Sham mice (open bars with open triangles); BLG mice (striped bars with filled diamonds); male C57BL/6J (blue); male BALB/cJ (red); female C57BL/6J (green); female BALB/cJ (orange). Bars indicate group average values ± SEM (n = 8–10 per group), two-way ANOVA.
To differentiate anxiety-like behavior from motor deficits, we also examined general locomotor activities of mice, the total distance traveled, and total time immobile. There were significant inter-strain differences between sex- and treatment-matched groups, with BALB/cJ mice, regardless of sex or treatment, being overall less active than C57BL/6J and avoiding the center zone (Supplementary Material 3). Furthermore, BLG-sensitized male BALB/cJ mice traveled less distance than strain-matched sham mice, although the difference in time immobile was not statistically significant. In contrast, there were no significant differences between sex-matched C57BL/6J sham and BLG groups, suggesting that decreases in the number of entries to the center and time spent in the center observed in male mice of this strain were not likely due to reduced mobility.
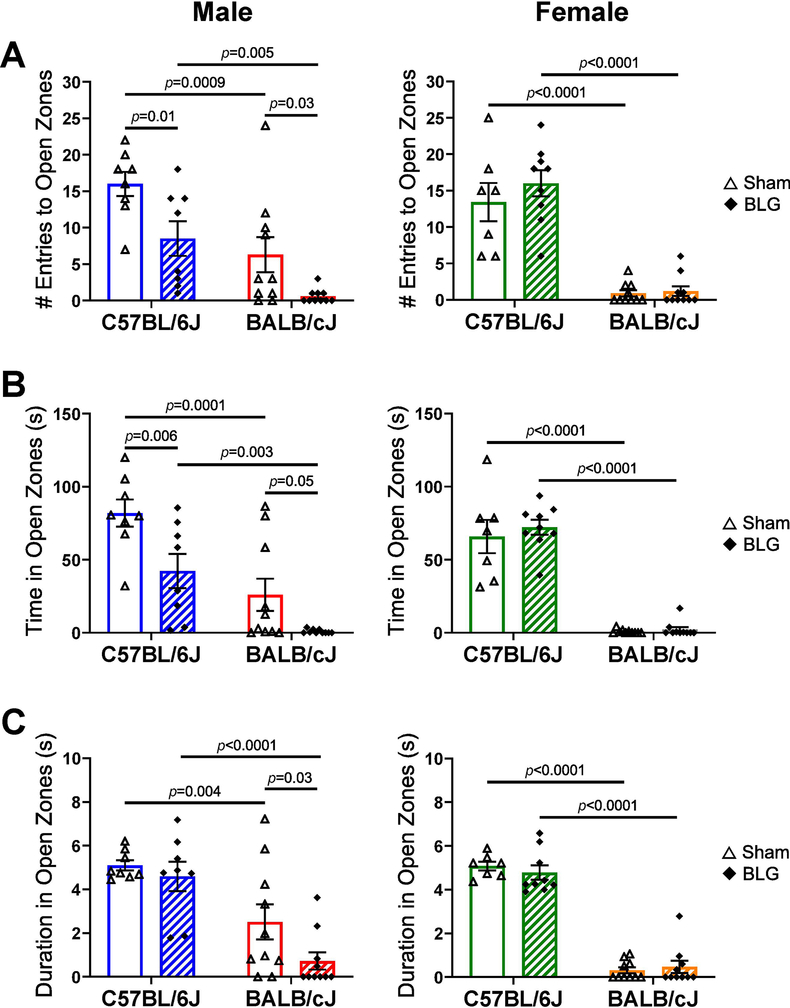
Anxiety-like behavior of mice was also assessed using the EZM test by monitoring their avoidance of the open zones (Fig. 5). As observed with the OF test, the BALB/cJ strain exhibited overall greater tendency to avoid open zones of the EZM compared to sex- and treatment-matched C57BL/6J mice. When comparing sex- and strain-matched groups, BLG-sensitized male mice of both strains made fewer entries to the open zones than their respective sham counterparts (Fig 5A; male C57BL/6J sham: 16 ± 2, n = 8; male C57BL/6J BLG: 9 ± 2, n = 8, p = 0.01; male BALB/cJ sham: 6 ± 2, n = 10; male BALB/cJ BLG: 0.6 ± 0.3, n = 10, p = 0.03). Similarly, BLG-sensitized male C57BL/6J and BALB/cJ mice spent less time in the open zone than sex- and strain-matched sham mice (Fig 5B; male C57BL/6J sham: 82 ± 9, n = 8; male C57BL/6J BLG: 42 ± 12, n = 8, p = 0.006; male BALB/cJ sham: 26 ± 11, n = 10; male BALB/cJ BLG: 0.9 ± 0.4, n = 10, p = 0.05). In addition, sensitized BALB/cJ male mice also spent less time in the open zone per entry than sham mice (Fig 5C; male BALB/cJ sham: 2.5 ± 0.8, n = 10; male BALB/cJ BLG: 0.7 ± 0.4, n = 10, p = 0.03). No differences were found in female mice of either strain in these parameters. These results further supported that sex and strain influenced the manifestation of CMA-associated anxiety-like behavior.
Figure 5. Elevated zero maze test.
Anxiety-like behavior of mice was observed on an EZM apparatus one day after the BLG challenge. The number of entries to the open zones (A), total time spent in the open zones (B), and the duration of each entry to the open zones (C) were quantified. Sham mice (open bars with open triangles); BLG mice (striped bars with filled diamonds); male C57BL/6J (blue); male BALB/cJ (red); female C57BL/6J (green); female BALB/cJ (orange). Bars indicate group average values ± SEM (n = 7–10 per group), two-way ANOVA.
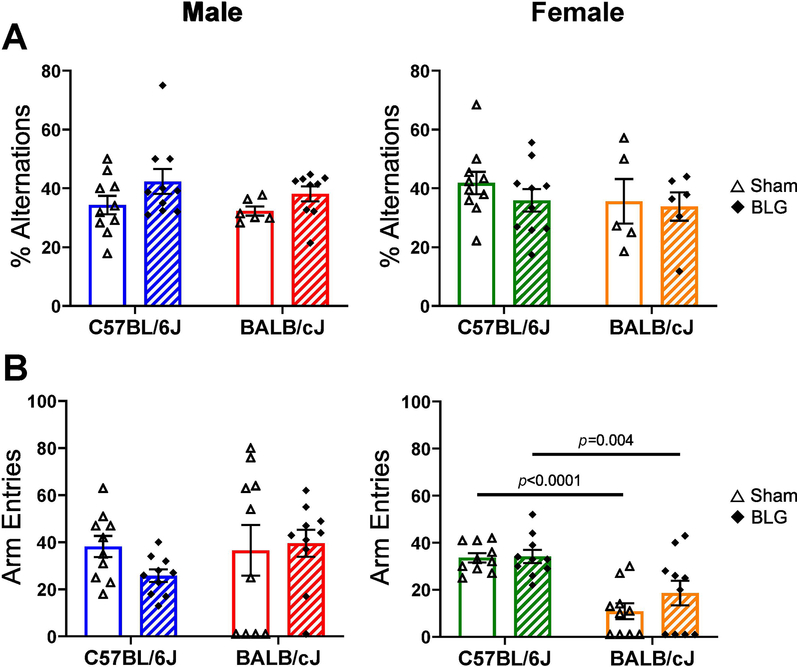
To evaluate whether CMA altered cognitive function, spatial memory was tested by quantifying the number of spontaneous alternations performed in the cross-maze test. In contrast to anxiety-like behavior, the ability of mice to strategically explore each arm of the maze was not affected by BLG sensitization in either strain or sex (Fig. 6A). However, it is important to note that many of the female BALB/cJ mice (5 sham and 6 BLG mice) either stayed in the entry arm or did not complete a cycle into all arms during their test period (Fig 6B) and were therefore excluded from the final analysis of % alternations (Fig. 6A). These results suggested that BLG sensitization did not affect cognitive ability with respect to spatial memory. No obvious strain differences were detected in their ability to alternately explore all arms of the maze.
Figure 6. Cross maze test.
Spatial memory of mice was tested using a cross maze one day after the BLG challenge. Mice were allowed to explore the maze freely for 12 min, and the numbers of successful sequence alternations (A) and the total number of entries into the arms (B) were recorded manually from video files. The number of successful alternations of arm entries made by each mouse was converted to percent alternations using the equation described in the Materials and Methods section. Mice that did not leave the starting position were removed from the final analysis. Sham mice (open bars with open triangles); BLG mice (striped bars with filled diamonds); male C57BL/6J (blue); male BALB/cJ (red); female C57BL/6J (green); female BALB/cJ (orange). Bars indicate group average values ± SEM (n = 5–10 per group), two-way ANOVA.
3.4. BLG sensitization yielded distinct sex- and strain-dependent plasma cytokine and chemokine profiles.
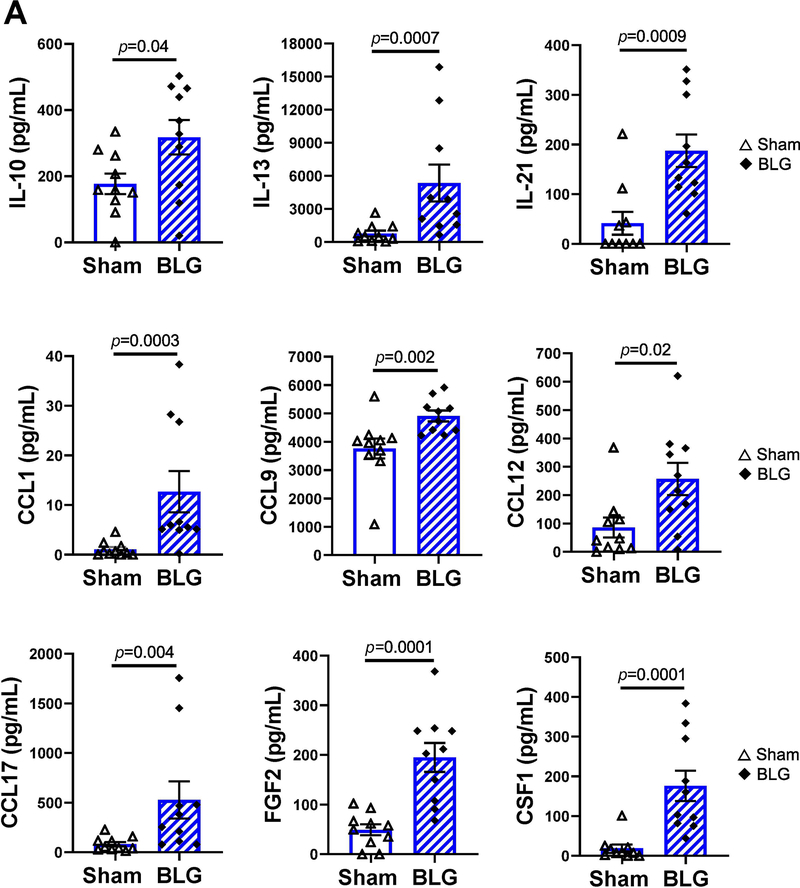
Because CMA-induced immunoglobulin production and anxiety-like behavior manifestation were sex- and strain-dependent, it was likely that distinct immune responses with unique inflammatory mediators were triggered in each mouse group upon allergen challenge. Thus, we next characterized cytokines, chemokines, and associated immunologic factors in each experimental group. In particular, we expected to observe elevation of Th1- and Th2-associated cytokines in C57BL/6J and BALB/cJ strains as they reportedly have respective immune biases (Autenrieth et al., 1994; Nishimura et al., 1997; Mills et al., 2000; Watanabe et al., 2004). To quantify the factors at the systemic level, we assessed the plasma samples using Quantibody® Mouse Cytokine Array Q5 system (Fig. 7, see Supplementary Material 4 for the complete quantitative data). In BLG-sensitized male C57BL/6J mice, 9 analytes were significantly increased in comparison to their respective sham mice (Fig 7A). CCL1 (C-C motif chemokine ligand 1) and CSF1 (colony stimulating factor 1, also known as macrophage colony stimulating factor or M-CSF) showed the most striking changes with allergen sensitization by increasing 12 ± 4 fold and 9 ± 2 fold, respectively. However, the absolute amounts of these factors were relatively low (CCL1 in sham: 1.1 ± 0.5 pg/mL, BLG: 13 ± 4 pg/mL; n = 10, p = 0.0003; CSF1 in sham: 19 ± 10 pg/mL, BLG: 176 ± 38 pg/mL; n = 10, p = 0.0001). Other analytes that were significantly induced in sensitized male C57BL/6J mice were, in the order of greatest to lowest, 7 ± 2 fold for IL-13 (sham: 780 ± 261 pg/mL, BLG: 5353 ± 1667 pg/mL; n = 10,p = 0.0007), 6 ± 2 fold for CCL17 (sham: 83 ± 22 pg/mL, BLG: 528 ± 187 pg/mL; n = 10,p = 0.004), 4.5 ± 0.8 fold for IL-21 (sham: 41 ± 23 pg/mL, BLG: 188 ± 33 pg/mL; n = 10, p = 0.0009), 4.0 ± 0.6 fold for FGF2 (sham: 49 ± 11 pg/mL, BLG: 195 ± 29 pg/mL; n = 10,p = 0.0001), 3.0 ± 0.7 fold for CCL12 (sham: 86 ± 35 pg/mL, BLG: 258 ± 57 pg/mL; n = 10, p = 0.02), 1.8 ± 0.3 fold for IL-10 (sham: 177 ± 31 pg/mL, BLG: 318 ± 52 pg/mL; n = 10, p = 0.04), and 1.31 ± 0.05 fold for CCL9 (sham: 3762.313 ± 355 pg/mL, BLG: 4910 ± 191 pg/mL; n = 10, p = 0.002). None of the analytes quantified with this assay were induced in BLG-sensitized female C57BL/6J mice (see Supplementary Material 4).
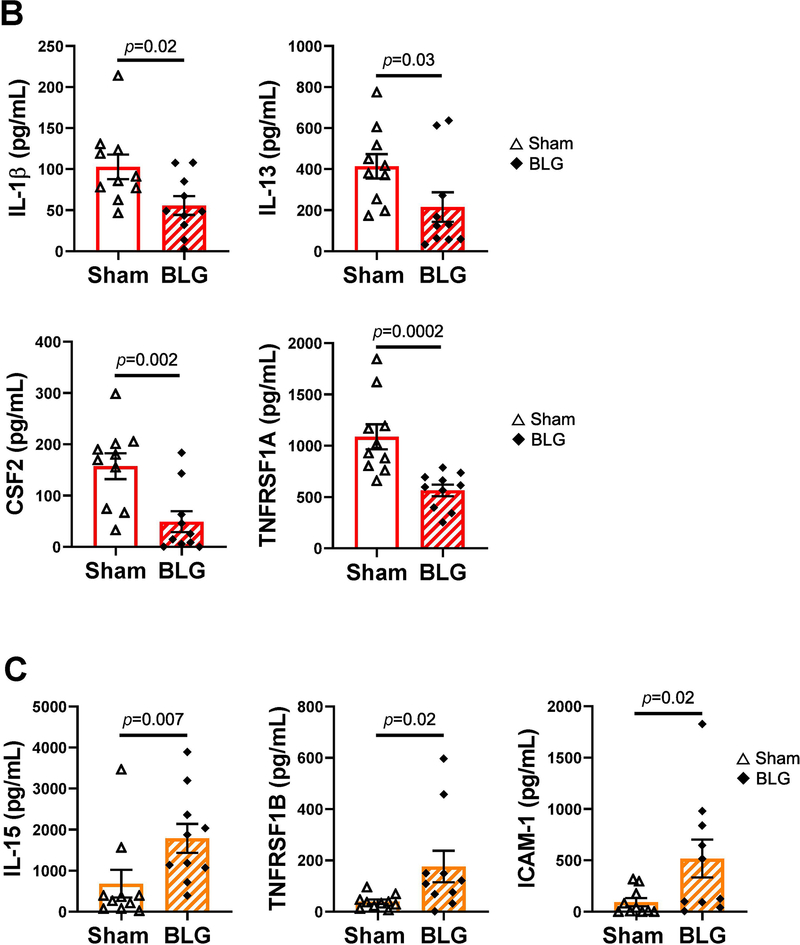
Figure 7. Plasma levels of immune mediators that were significantly different between sex- and strain matched sham and BLG-sensitized mice.
Levels of the 40 immune mediators included in the Quantibody Mouse Cytokine Array 5 (QAM-CYT-5) were quantified from plasma samples. Only the analytes that showed significant differences between sex- and strain-matched sham and BLG groups are shown for C57BL/6J male mice (A), BALB/cJ male mice (B), and BALB/cJ female mice (C). No significant differences in any of the detected analytes were observed in female C57BL/6J mice (not shown). The quantification of all analytes for all mouse groups are presented as Supplementary Material 4. Sham mice (open bars with open triangles); BLG mice (striped bars with filled diamonds); male C57BL/6J (A); male BALB/cJ (B); female BALB/cJ (C). Bars indicate group average values ± SEM (n = 10 per group), Mann-Whitney U test.
The cytokine profile of BLG-sensitized male BALB/cJ mice was distinct from that of sex-matched C57BL/6J mice, and 4 analytes were significantly reduced in BLG-sensitized mice compared to the sham mice (Fig 7B). The analyte levels were lower by 0.5 ± 0.1 fold for IL-1β (sham: 103 ± 15 pg/mL, BLG: 56 ± 11 pg/mL, n = 10, p = 0.02), 0.5 ± 0.2 fold for IL-13 (sham: 414 ± 59 pg/mL, BLG: 216 ± 72 pg/mL; n = 10, p = 0.03) 0.3 ± 0.1 fold for CSF2 (sham: 157 ± 25 pg/mL, BLG: 49 ± 20 pg/mL, n = 10, p = 0.002), and 0.52 ± 0.05 fold for TNFRSF1A (sham: 1087 ± 121 pg/mL, BLG: 566 ± 56 pg/mL; n = 10, p = 0.0002) in BLG sensitized mice. In contrast, 3 analytes were increased in BLG-sensitized female BALB/cJ mice (Fig 7C), including 2.6 ± 0.5 fold for IL-15 (sham: 683 ± 340 pg/mL, BLG: 1788 ± 353 pg/mL, n = 10, p = 0.007), 5 ± 2 fold for TNFRSF1B (sham: 39 ± 8 pg/mL, BLG: 176 ± 61 pg/mL, n = 10, p = 0.02), and 6 ± 2 fold for ICAM-1 (sham: 93 ± 40 pg/mL, BLG: 518 ± 184 pg/mL, n = 10, p = 0.02).
This array data demonstrated that BLG sensitization resulted in altered levels of distinct sets of immunologic mediators in the circulation, with C57BL/6J male mice having the greatest number of mediators that were significantly affected. Therefore, our results indicated that the same allergen triggered varying immune responses depending on the sex and strain of sensitized mice. Importantly, the cytokine profiles from BLG-sensitized C57BL/6J and BALB/cJ mice failed to categorize their strain-specific responses simply into Th1 and Th2 responses, respectively.
3.5. BLG-sensitization differentially altered the composition of intestinal microbial community in a sex- and strain-specific manner.
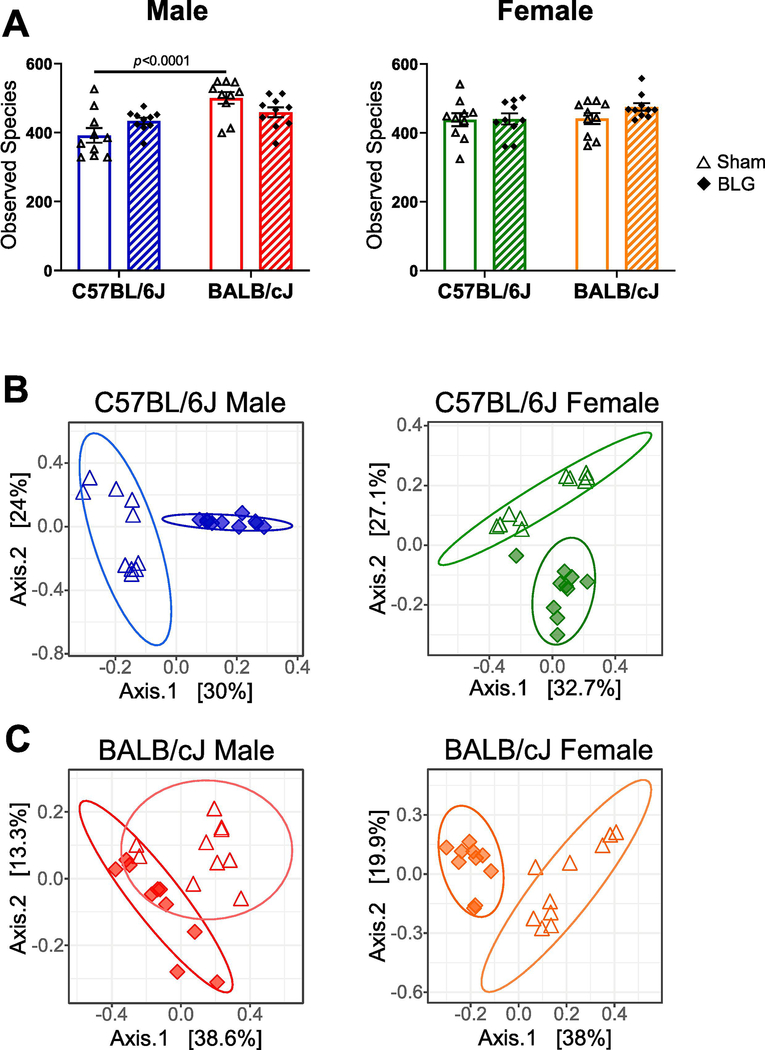
Because BLG was orally introduced during the sensitization procedure, we postulated that allergen sensitization and the subsequent challenge had produced inflammatory conditions in the intestinal tract and influenced the commensal microbial community. Altered intestinal microbiota has been implicated in a variety of pathological conditions, including food allergy and neuropsychiatric disorders (Wang et al., 2011; Scheperjans et al., 2015; Blazquez and Berin, 2017; Vuong et al., 2017; Pulikkan et al., 2018). To assess whether BLG sensitization resulted in alterations in intestinal microbiota, we next performed microbiome analysis by 16S ribosomal RNA gene sequencing from stool samples. Following sequencing, microbial taxonomy was classified using amplicon sequence variants (ASVs) at the species level. Approximately 400–500 species were classified in each treatment group (Fig. 8A). There was a significant strain-dependent difference in the number of observed species between C57BL/6J and BALB/cJ male sham mice (Fig. 8A). Species richness was greater for BALB/cJ sham mice compared to respective C57BL/6J mice in males (C57BL/6J sham: 392.2 ± 21.3, BALB/cJ sham: 500.3 ± 17.0; n = 10, p < 0.0001). Furthermore, assessment of alpha diversity indicated significant differences between sex- and strain-matched treatment groups for male C57BL/6J mice (Simpson index) and female BALB/cJ mice (Shannon and Simpson indices), showing BLG-associated decrease and increase in biodiversity, respectively (Supplementary Material 5).
Figure 8. Effects of BLG sensitization on fecal microbiome.
Microbial DNA was isolated from fecal pellets and 16S ribosomal RNA gene sequencing was performed. Fecal microbial compositions were determined as described in the Materials and Methods section, and alpha and beta diversities were assessed. (A) Assessment of alpha diversity with the number of observed species. Sham mice (open bars with open triangles); BLG mice (striped bars with filled diamonds). Bars indicate group average values ± SEM (n = 10 per group), two-way ANOVA. (B, C) Assessment of beta diversity with Bray-Curtis principal coordinate analysis. Sham mice (open triangles); BLG mice (filled diamonds). Male C57BL/6J (blue); male BALB/cJ (red); female C57BL/6J (green); female BALB/cJ (orange).
We next performed PCoA using the Bray-Curtis dissimilarity method to cluster the beta diversity of sex- and strain-matched treatment groups. There was clear separation between sham and BLG groups in both male and female C57BL/6J mice (Fig. 8B). Similarly, the clustering of the two groups was distinct for female BALB/cJ mice, whereas some overlap was observed for male BALB/cJ mice (Fig. 8C). These results suggested that intestinal microbial composition becomes altered with BLG sensitization, although the extent of the change may be strain-dependent.
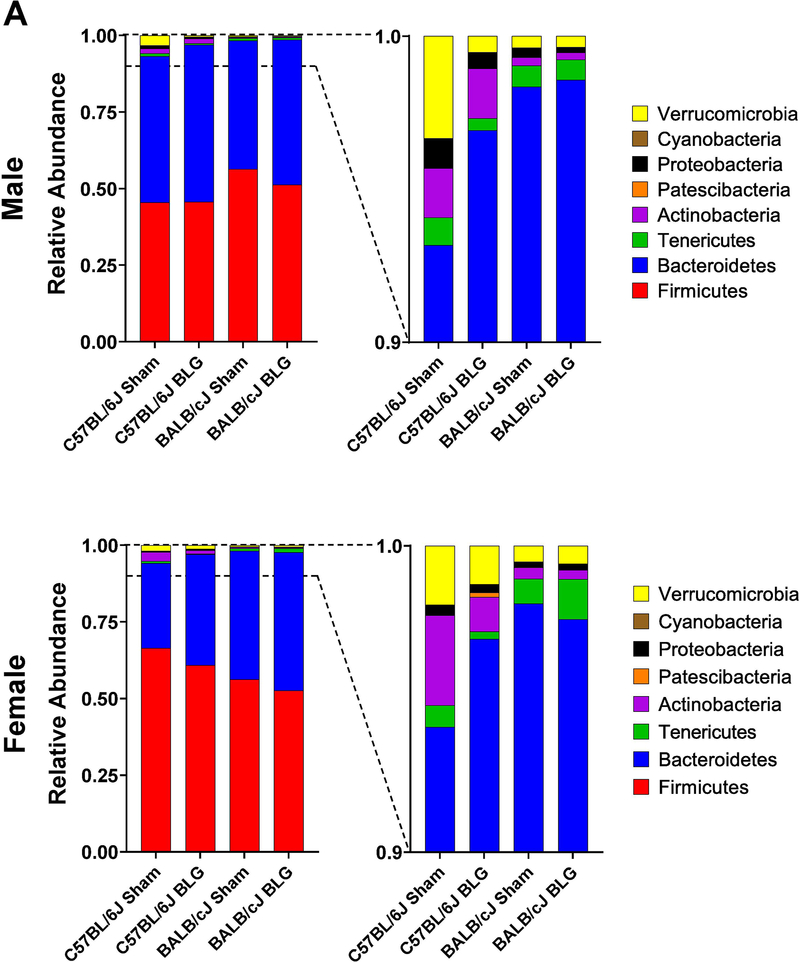
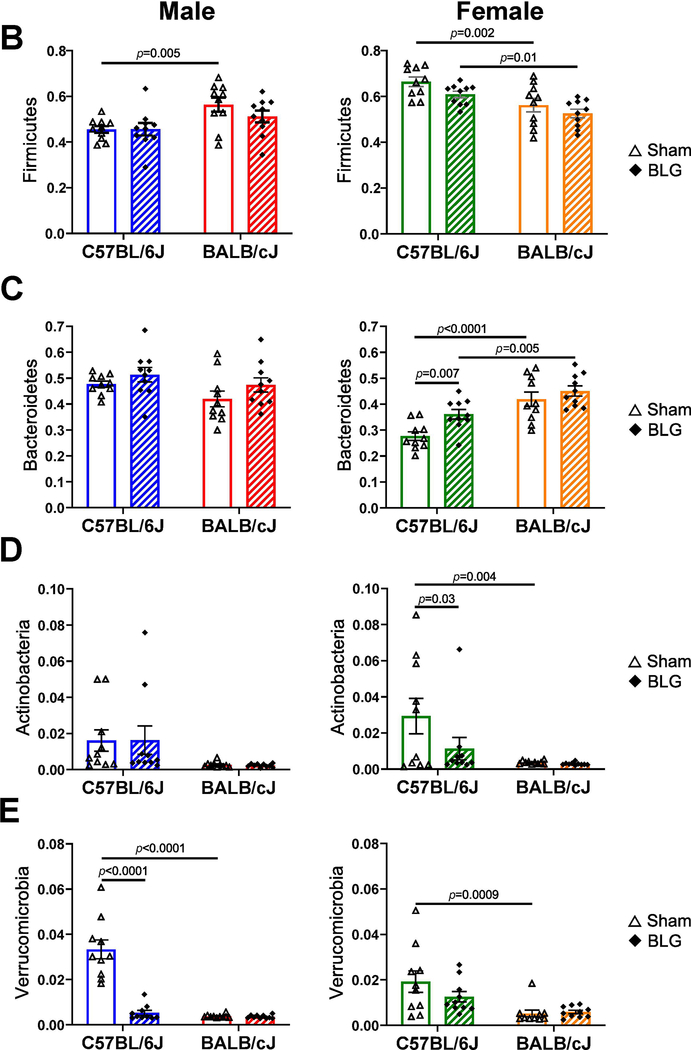
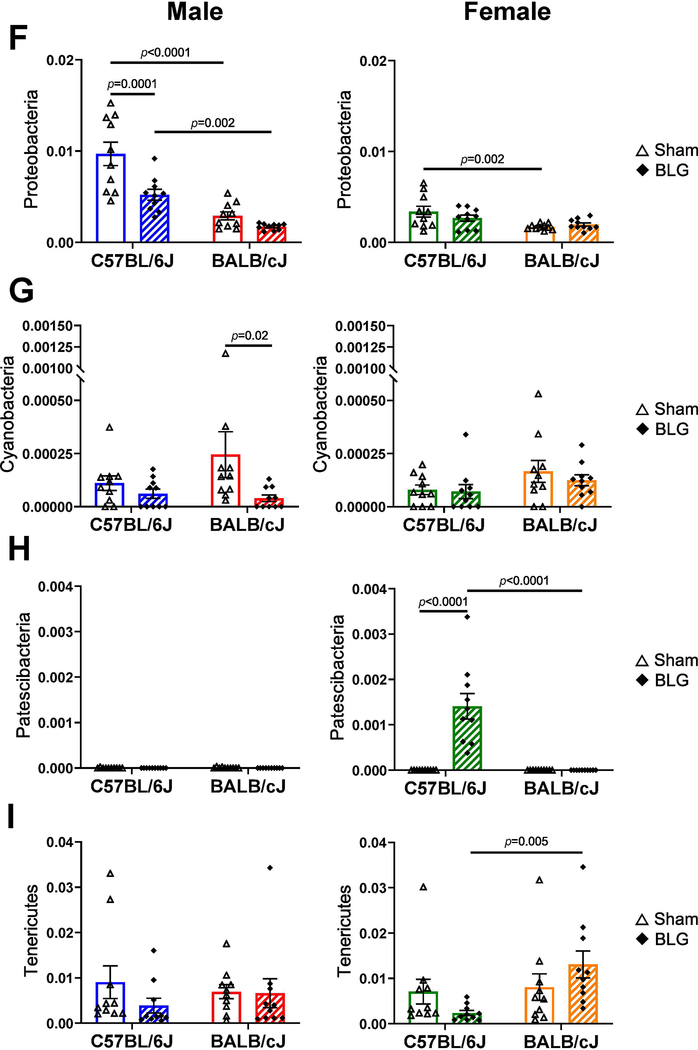
When taxonomic compositions from the experimental groups were compared at the phylum level, each group showed a distinct profile, although Firmicutes and Bacteroidetes were the two dominant bacterial phyla as reported elsewhere (Fig. 9A). The relative abundance of these phyla was uniquely influenced by BLG sensitization in a sex- and strain-dependent manner (Fig. 9B–I). For C57BL/6J, Verrucomicrobia and Proteobacteria were significantly lower in BLG-sensitized male mice, whereas sensitization resulted in a greater abundance of Bacteroidetes and Patescibacteria and reduced abundance of Actinobacteria in female mice. In contrast, for BALB/cJ mice, Cyanobacteria was the only phylum that was lower in BLG-sensitized male mice, while female mice did not show any significant differences between sham and BLG groups in the identified phyla. Among the differences described above, the most notable sensitization-associated differences were reduced abundance of Verrucomicrobia in male C57BL/6J by 84.1% (0.16 ± 0.03 fold) and increased abundance of Bacteroidetes in female C57BL/6J by 30.5% (1.31 ± 0.07 fold). The complete microbiome profiles for all the experimental groups are provided in Supplementary Material 6.
Figure 9. Sensitization-associated differences in the relative abundance of major bacterial phyla detected from fecal microbiome analysis.
Microbial DNA was isolated from fecal pellets and 16S ribosomal RNA gene sequencing was performed. (A) Relative abundances of detected bacterial phyla were compared among sex-matched groups. (B-I) Relative abundance of each of the major phyla was compared to their sex-matched groups. Sham mice (open bars with open triangles); BLG mice (striped bars with filled diamonds); male C57BL/6J (blue); male BALB/cJ (red); female C57BL/6J (green) female BALB/cJ (orange). Bars indicate group average values ± SEM (n = 10 per group), two-way ANOVA.
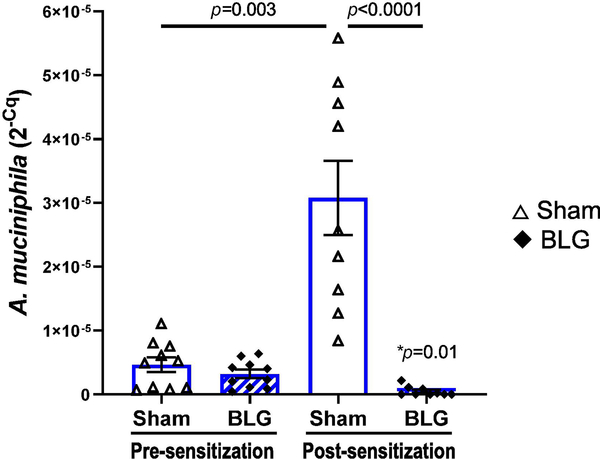
3.6. Proliferation of Akkermansia muciniphila in C57BL/6J male mice was inhibited with BLG sensitization.
Because C57BL/6J male mice exhibited significant changes in intestinal microbiota associated with anxiety-like behavior, we focused on this group to investigate the potential role of their commensal bacteria in their behavioral and immunologic responses. Here, we examined the pre- and post-sensitization fecal amounts of Akkermansia muciniphila, a species belonging to the phylum, Verrucomicrobia, which was significantly decreased in BLG-sensitized mice (Fig. 9E). A. muciniphila has also been implicated in various disease conditions, including neurological disorders (Wang et al., 2011; Hill-Burns et al., 2017; Li et al., 2019; Xu et al., 2019). As shown in Fig. 10, the relative amounts of A. muciniphila detected in pre-sensitization samples from the sham and BLG groups were comparable, ranging from 4.5×10−7 to 1.1×10−5 (sham average: 4×10−6 ± 1×10−6, BLG average: 3.2×10−6 ± 0.7×10−6; expressed as 2−Cq values). However, A. muciniphila increased in sham mice during the course of the 5-week sensitization period, averaging 22 ± 8-fold increases at post-sensitization with a range of 2.2 to 73.5-fold increases. On the other hand, all BLG-sensitized mice, except one mouse identified as an outlier (2−Cq value: 9.4×10−6, see the Materials and Methods section), showed profound decreases in the relative amount of the bacteria after sensitization, averaging 0.2 ± 0.1 fold, indicating that the growth of this bacteria was stifled in this group. Because A. muciniphila was the predominant species of Verrucomicrobia identified and detected in the microbiome (Supplementary Material 6), the significant reduction of the phylum observed in BLG-sensitized C57BL/6J male mice is therefore likely due to attenuated colonization of A. muciniphila (Fig. 9A).
Figure 10. Differences in the amount of A. muciniphila in sham and BLG-sensitized male C57BL/6J mice before and after the sensitization procedure.
Microbial DNA samples were isolated from fecal pellets that had been collected before (pre-sensitization) and after (post-sensitization) the 5-week sensitization procedure. The amount of A. muciniphila in each of the DNA samples were quantified by qPCR using a specific primer pair. Sham mice (open bars with open triangles); BLG mice (striped bars with filled diamonds). Bars indicate group averages of individual 2−Cq values ± SEM (n = 10 per group), sham vs. BLG: two-way ANOVA; treatment-matched pre- vs post-sensitization values were compared using paired t-test. *p=0.01 indicates comparison between pre- and post-sensitization BLG.
3.7. The altered microbiome profile of BLG-sensitized male C57BL/6J was associated with molecular interactions known to affect neurological functions.
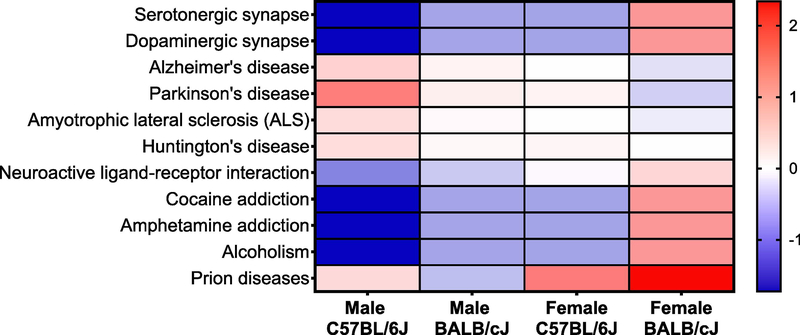
In order to explore possible molecular targets that might have been influenced in our BLG-sensitized mice, we identified the pathways that were associated with the microbial changes observed in this study using the Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Database (https://www.genome.jp/kegg/pathway.html). Known molecular interaction pathways with significant differences between sex- and strain-matched sham and BLG-sensitized groups are presented as Supplementary Material 7. We found a greater number of pathways that were significantly associated with the microbiome of BLG-sensitized C57BL/6J male mice than any other groups. Interestingly, among them were some pathways involved in neurological disorders and neurotransmission, such as serotonergic/dopaminergic synapses and Parkinson’s disease (Fig 11). Although the analysis does not articulate specific molecules within each pathway that were likely affected by the sensitization-associated microbiome changes in C57BL/6J male mice, the result suggests that the neurological functions were possibly influenced in these mice.
Figure 11. Central nervous system-related pathways associated with the changes in microbiota in BLG-sensitized mice.
KEGG pathway analysis was used to identify known functional pathways that were significantly associated with the microbiome profiles in our mouse groups. Only the pathways that are related to the central nervous system functions are shown. All results from the KEGG pathway analysis are provided in Supplementary Material 7.
4. DISCUSSION
The severity and presentations of food allergy symptoms widely vary among individuals and likely contribute to inconsistencies across human cohort studies that investigate the association of food allergy with affective, behavioral, and cognitive disorders. Indeed, the heterogeneity in responses to allergens is often observed in human patients (Burks et al., 2012; Bird et al., 2015; Sicherer and Sampson, 2018; Fritscher-Ravens et al., 2019). We postulated that the presence of allergy-associated behavioral manifestations would be influenced by sex and genetic background. In this study, we therefore used a mouse model of CMA to evaluate the effect of strain and sex on CMA-associated anxiety-like behavior and cognitive function as well as physical reactions, immunological responses, and microbial changes.
We first assessed whether the BLG sensitization regimen effectively induced CMA. Following an oral allergen challenge, both C57BL/6J and BALB/cJ strain mice showed no or mild observable reactions, scoring a severity level of 0–2 upon allergen challenge (Fig. 2B). This outcome was not unexpected given that we and others have previously reported mild responses to allergen challenges with these strains (Marco-Martin et al., 2017; Germundson et al., 2018; Smith et al., 2019), and C57BL/6 and BALB/c backgrounds are known to be more resilient to experimental allergic sensitization than other strains (Xu et al., 2018). However, greater numbers of BLG-sensitized BALB/cJ mice scored 2 than sex- and treatment-matched C57BL/6J mice, rendering the modest differences in the clinical scores and body temperature from their respective sham groups statistically significant (Fig. 2B, 2C). Mast-cell-derived histamine is a known contributor of allergy-induced hypothermia and respiratory distress, acting via H1/H2 histaminergic receptors. The absence of mast cells or histamine production in knockout mice, as well as histaminergic receptor antagonists in wild-type mice, have been shown to ameliorate these symptoms after inducing passive systemic anaphylaxis (Makabe-Kobayashi et al., 2002).
Thus, our observation suggested that BLG-sensitized BALB/cJ mice were more susceptible to mast cell degranulation upon allergen challenge. As for the allergen-specific immunoglobulins, the sensitization-induced changes in their levels did not closely mirror the physiological responses, and all BLG groups, except C57BL/6J females, showed small but significant increases in allergen-specific IgE (Fig. 3A). Furthermore, IgG isotypes were also elevated in both male and female BALB/cJ mice after sensitization (Fig. 3B and 3C). In contrast, we did not observe elevated IgG1 in the BLG groups of C57BL/6J mice. These results indicated that the production of allergen-specific immunoglobulin was differentially affected by the strain, particularly highlighting the difference in the amounts of IgG1 production. However, we have previously observed elevated BLG-specific IgG1 in male and female C57BL/6J mice in our earlier study, in which mice were challenged twice, 1 week apart (Smith et al., 2019). Thus, the number of allergen exposure and/or duration after sensitization may also affect the amounts of allergen-specific immunoglobulins.
A limitation to note for the measurement of allergen-specific IgE in the circulation is that it may not precisely reflect the total amount of the IgE produced by an allergic individual, since produced IgE becomes rapidly associated with high-affinity Fcε receptor I on immune cells such as mast cells and basophils, and free IgE molecules are subjected to rapid degradation (Lawrence et al., 2017). Moreover, the serum samples we used in this study were prepared from the terminal blood, which was collected after 1 week from the last sensitization dose and 2 days from the allergen challenge (see Fig. 1A). The half-life of IgE is estimated to be 2–3 days in humans (Lawrence et al., 2017) and 12 hours in mice (Vieira and Rajewsky, 1988), and thus, the amounts of IgE detected in our assay may have been an underestimation of actual amounts produced. Another limitation of our current study was that IgG2a was measured as one of the allergen-specific immunoglobulins. However, C57BL/6J mice do not produce IgG2a but instead produce IgG2c isotype (Jouvin-Marche et al., 1989). For further assessments for allergen-induced immunoglobulin levels in this strain, an IgG2c-specific assay should be used.
Despite the lack of anaphylaxis and other overt physical indications of severe allergic responses, BLG-sensitized C57BL/6J mice exhibited anxiety-like behavior one day after the allergen challenge. This CMA-associated behavior change was not observed in female C57BL/6J mice, an outcome in line with our previous study (Smith et al., 2019). Although the results from the OF test were difficult to compare in BALB/cJ mice due to their overall inactivity, the differences between sham and BLG mice were detected for male mice by the number of fecal pellets and EZM (Fig. 4D and 5A–C). Again, these differences were not observed in BALB/cJ female mice, suggesting that females were less inclined to exhibit CMA-associated anxiety-like behavior regardless of strain. The cross-maze test did not show significant differences between sham and BLG mice in any of the groups or strain- or sex-dependent effects, suggesting that BLG sensitization followed by acute exposure to the allergen did not affect cognitive function, at least for spatial memory (Fig. 6).
Similar male-biased symptom manifestations have also been reported in human patients. Food allergy and certain types of behavioral conditions, such as ADHD and autism, are more commonly diagnosed in young males (Polanczyk et al., 2007; Kim et al., 2011; Pinares-Garcia et al., 2018). The sex-dependent dichotomy may be explained by the differences in the number of T lymphocytes and the presence of promoter elements within immune-related genes that can be regulated by sex hormones (DunnGalvin et al., 2006; Markle and Fish, 2014; Klein and Flanagan, 2016; Laffont and Guery, 2019). In rodent studies, the contribution of estrogen to the regulation of anxiety-like behavior has also been investigated, although anxiolytic effects seem to depend on the dose of the hormone, behavior tests used, and age of mice (Boivin et al., 2017; Borrow and Handa, 2017). While it was outside the scope of our current study, identification of mechanistic or molecular factor(s) that protected our female mice from manifesting CMA-induced anxiety-like behavior may provide potential therapeutic targets for anxiety.
Sex- and strain-specific variations were also found in the number, type, and levels of immune mediators detected in plasma, with male C57BL/6J mice showing the most number of analytes that were significantly altered with BLG sensitization, followed by male and female BALB/cJ mice (Fig. 7A–C). No significant changes in plasma mediator levels were detected in female C57BL/6J mice, again underscoring the resilience of this group to the sensitization. In C57BL/6J males, increases in helper T cell (Th) associated cytokines and chemokines were particularly notable. While the C57BL/6 and BALB/c strains are typically described to have Th1- and Th2-biased immune responses, respectively (Autenrieth et al., 1994; Nishimura et al., 1997; Mills et al., 2000; Watanabe et al., 2004), it has been argued that this generalization is based on infection models and does not apply to allergy paradigms (HayGlass et al., 2005). Our results also seemed to support this argument and did not categorize the responses of the two strains strictly into either Th1 or Th2. Instead, significant increases in IL-13 and IL-21 suggested that the responses of BLG-sensitized C57BL/6J mice were likely mediated by follicular helper T cells (Tfh), which are crucial for the production of IgE and other isotypes via differentiation of B cells (Gowthaman et al., 2019, also reviewed by Yao et al., 2020).
The mediator responses by male C57BL/6J and BALB/cJ mice were clearly contrasting, with the former males showing increases in some mediators and the latter exhibiting decreases in a distinct set of mediators. In particular, sensitization-associated changes in IL-13 were observed in both strains but in opposite directions (Fig. 7A&B). Although the functional significance of these CMA-induced differential changes in the analytes is yet to be determined, the elevation of IL-13 in C57BL/6J males likely facilitated the production of BLG-specific IgE. Concurrent increases in IL-10, FGF2, CSF1, and CCL chemokines in C57BL/6J males also suggested that mobilization and/or proliferation of immune cells were prompted, triggering complex systemic pro- and anti-inflammatory responses in BLG-sensitized mice of this strain. It should be noted that some of these cytokines had been reported to be elevated in mouse models with more severe allergic reactions. For example, CCL1 and CCL17 were induced in the intestines of ovalbumin (OVA)-sensitized mice that developed diarrhea with mast cell infiltration (Knight et al., 2007), and high levels of CCL9 (MIP-1γ) was found in OVA-sensitized mouse lungs with increased airway resistance and eosinophil infiltrates (Rose et al., 2010). These results suggested that, despite their lack of overt allergic reactions, BLG-sensitized C57BL/6J mice elicited similar immune responses to those of other animal models of severe allergies, and thus, the absence of typical allergic reactions might not necessarily indicate the absence of hypersensitivity to the allergen. Following the same line of argument, the changes in the plasma mediators may not be accurate indicators of CMA-associated behavioral manifestations. As mentioned above, the cytokine profile of BALB/cJ male mice was conflicting with their C57BL/6J counterpart while both groups displayed anxiety-like behavior when assessed with the EZM test. In addition, a few of the inflammatory mediators were elevated in BALB/cJ female mice after sensitization (Fig. 7C), but this group did not exhibit behavioral abnormalities, at least with the tests we performed.
It may also be argued that the observed cytokine changes were reflective of different stress levels mice might have experienced from the behavior test one day before their blood was collected. Indeed, it has been shown that foot-shock stress increases the amounts of inflammatory cytokines in mice, including IL-10 and IL-13 (Cheng et al., 2015). However, the plasma levels of these cytokines reportedly return to the control levels after 24 h (Cheng et al., 2015), rendering the possibility that stress was a major contributor of the cytokine/chemokine changes less likely. Taken together, testing for the altered plasma levels of immune mediators may be more sensitive in detecting the development of allergen hypersensitivity than the presence of physical reactions, although it may not predict the presence of allergen-induced behavioral abnormality.
We then postulated that intestinal microbiota would be altered following BLG sensitization procedure. Altered microbiota, or dysbiosis, has been reported in individuals with food allergy as well as with neuropsychiatric disorders (Bunyavanich et al., 2016; Cenit et al., 2017; Savage et al., 2018). In addition, a growing amount of evidence supports that microbiota influences behavior and mood (Tomova et al., 2015; Aizawa et al., 2016; Mangiola et al., 2016). Indeed, we found clear differences in beta diversity at the phylum level between sham and BLG-sensitized mice in all groups, although the most notable change with BLG sensitization was a marked decrease in Verrucomicrobia (Fig. 8 & 9). While the number of observed species remained relatively unchanged (Fig. 8A), Simpson index for C57BL/6J male mice indicated decreased biodiversity, supporting previous findings in humans that changes in alpha diversity was associated with milk allergy (Berni Canani et al., 2018; Shen et al., 2019). We further demonstrated that A. muciniphila was significantly lowered in the BLG-sensitized male C57BL/6J mice while its amount was increased in sham mice during the course of sensitization (Fig. 10). This observation suggested that the colonization of the bacteria in the intestines was restricted during allergy development. A. muciniphila has been reported to be an important commensal inhabitant that protects epithelial barrier integrity by regulating mucus production in the host (Reunanen et al., 2015). Furthermore, altered relative abundance of A. muciniphila has been found in obesity, diabetes, inflammatory bowel disease as well as neurological diseases such as Parkinson’s disease, and autism (Wang et al., 2011; Everard et al., 2013; Hill-Burns et al., 2017; Li et al., 2019; Xu et al., 2019). Thus, it is possible that the lack of A. muciniphila is associated with anxiety-like behavior and/or other behavioral manifestations. Further studies in which the amounts of intestinal A. muciniphila are experimentally manipulated prior to behavior testing will confirm this association. Our KEGG Pathway analysis further supported that the microbiome changes observed in BLG-sensitized male C57BL/6J mice likely influenced neurological functions, including those involved in Parkinson’s disease and dopaminergic and serotonergic synapses (Fig. 11). These pathways consist of many molecular interactions between enzymes, receptors, channels, transporters, etc., and therefore it is necessary to validate the involvement of specific pathway components that were indeed affected by the sensitization-induced dysbiosis. This extrapolated bioinformatics approach, however, provides valuable information in narrowing down potential targets for further investigation in our future studies.
5. CONCLUSIONS
Taken together, our results demonstrated some significant sex- and strain-dependent differences in the symptom presentations of experimentally induced CMA in mice, with males of both strains having the propensity to display anxiety-like behavior. Other sensitization-associated differential presentations included serum levels of allergen-specific IgE/IgG, plasma levels of immune mediators, and changes in microbiota compositions. We hereby provide evidence that the manifestations of hypersensitivity to the same allergen are influenced by genetic variables in individuals, and therefore diagnosing food allergy by immediate reactions after allergen challenge and immunoglobulin levels may exclude a population of individuals with milder or atypical responses. In addition, stratification of allergic cohorts with additional diagnostic criteria may reduce apparent inconsistencies in human studies.
Supplementary Material
S1. Serum levels of BLG-specific immunoglobulin isotypes. Terminal blood samples were used to detect BLG-specific serum IgE (A), IgG1 (B) and IgG2a (C) using ELISA. Optical density values at 450 nm were used to plot the graphs after subtracting the background values at 550 nm (OD450-550). Sham mice (open bars with open triangles); BLG mice (striped bars with filled diamonds); male C57BL/6J (blue); male BALB/cJ (red); female C57BL/6J (green); female BALB/cJ (orange). Bars indicate group average values in OD450-550 ± SEM (n = 10 per group), two-way ANOVA.
S2. Time-dependent changes in the parameter measurements in the open-field activity monitoring. Overall activities of male (S1A) and female (S1B) sham and BLG-sensitized mice in an open-field arena were recorded for 10 min, and the activity parameters indicated in the y-axes were quantified by ANY-maze software. The values were graphed in relation to time in minutes (x-axis) during the test duration. Sham: open triangles; BLG-sensitized: filled diamonds. Values indicate group average ± SEM (n = 8-10). *p < 0.05 when compared to the sham mice at the same time point by t-test.
S3. General locomotor activities recorded during the open-field activity monitoring. Total distance traveled and total time immobile were computed using ANY-maze software to assess potential effects of BLG sensitization on overall activities. Sham mice (open bars with open triangles); BLG mice (striped bars with filled diamonds); male C57BL/6J (blue); male BALB/cJ (red); female C57BL/6J (green) female BALB/cJ (orange). Bars indicate group average values ± SEM (n = 8-10 per group), two-way ANOVA.
S4. Complete cytokine/chemokine array data using Quantibody Mouse Cytokine Array 5.
S5. Extended diversity indices. In addition to the observed species, Chao1, Shannon, and Simpson indices were used to compare the diversity between sex-matched groups.
S6. Complete microbiome profiles of all experimental groups.
S7. Complete KEGG Pathway analysis performed based on the sensitization-induced microbiome changes. Tax4Fun2 reference database was used to identify differential activation of pathways based upon changes in microbiome. Level 1 pathway classifications with an adjusted p-value < 0.05 (t-test with Bonferroni correction) and average log2 greater or less than 1 (fold changes greater or less than 2) are displayed in a heatmap grouped by level 2 classification.
HIGHLIGHTS.
Sensitization of C57BL/6J and BALB/cJ mice to β-lactoglobulin resulted in mild hypersensitivity.
More BALB/cJ mice exhibited observable symptoms and hypothermia than C57BL/6J mice.
Sensitized male mice of both strains exhibited anxiety-like behavior after challenge.
The levels of immunologic factors were altered differentially by strain and sex in sensitized mice.
Allergic sensitization modified strain- and sex-specific gut microbiota profiles.
ACKNOWLEDGMENT
The authors thank Dr. Bony De Kumar and Ms. Hannah Ness for their assistance with sample quality control for 16S microbial DNA sequencing, the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with genomic analysis, and Drs. Harpreet Kaur and Mona Sohrabi-Thompson, and Mr. Jakson Martens for their technical assistance.
This study was supported by the National Institute of General Medical Sciences of the National Institutes of Health, grant numbers P20GM103442 and 5P20GM113123. Behavioral experiments were performed at the University of North Dakota Behavioral Research Core Facility partly supported by NIH P20GM103442 and U54GM128729.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acker WW, Plasek JM, Blumenthal KG, Lai KH, Topaz M, Seger DL, Goss FR, Slight SP, Bates DW, Zhou L (2017) Prevalence of food allergies and intolerances documented in electronic health records. J Allergy Clin Immunol 140:1587–1591 e1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afari N, Schmaling KB, Barnhart S, Buchwald D (2001) Psychiatric comorbidity and functional status in adult patients with asthma. J Clin Psychol Med Settings 8:245–252. [Google Scholar]
- Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, Ota M, Koga N, Hattori K, Kunugi H (2016) Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord 202:254–257. [DOI] [PubMed] [Google Scholar]
- Altemus M (2006) Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav 50:534–538. [DOI] [PubMed] [Google Scholar]
- Autenrieth IB, Beer M, Bohn E, Kaufmann SH, Heesemann J (1994) Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun 62:2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehler P, Chad Z, Gurbindo C, Bonin AP, Bouthillier L, Seidman EG (1996) Distinct patterns of cow’s milk allergy in infancy defined by prolonged, two-stage double-blind, placebo-controlled food challenges. Clin Exp Allergy 26:254–261. [PubMed] [Google Scholar]
- Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN (2007) Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav 86:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berin MC, Zheng Y, Domaradzki M, Li XM, Sampson HA (2006) Role of TLR4 in allergic sensitization to food proteins in mice. Allergy 61:64–71. [DOI] [PubMed] [Google Scholar]
- Berni Canani R, De Filippis F, Nocerino R, Paparo L, Di Scala C, Cosenza L, Della Gatta G, Calignano A, De Caro C, Laiola M, Gilbert JA, Ercolini D (2018) Gut microbiota composition and butyrate production in children affected by non-IgE-mediated cow’s milk allergy. Sci Rep 8:12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird JA, Lack G, Perry TT (2015) Clinical management of food allergy. J Allergy Clin Immunol Pract 3:1–11. [DOI] [PubMed] [Google Scholar]
- Blazquez AB, Berin MC (2017) Microbiome and food allergy. Transl Res 179:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blöndal V, Malinovschi A, Sundbom F, James A, Middelveld R, Franklin KA, Lundbäck B, Janson C (2020) Multimorbidity in asthma, association with allergy, inflammatory markers and symptom burden, results from the Swedish GA(2) LEN study. Clin Exp Allergy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin JR, Piekarski DJ, Wahlberg JK, Wilbrecht L (2017) Age, sex, and gonadal hormones differently influence anxiety- and depression-related behavior during puberty in mice. Psychoneuroendocrinology 85:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boris M, Mandel FS (1994) Foods and additives are common causes of the attention deficit hyperactive disorder in children. Ann Allergy 72:462–468. [PubMed] [Google Scholar]
- Borrow AP, Handa RJ (2017) Estrogen receptors modulation of anxiety-like behavior. Vitam Horm 103:27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA (2010) Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunyavanich S, Shen N, Grishin A, Wood R, Burks W, Dawson P, Jones SM, Leung DYM, Sampson H, Sicherer S, Clemente JC (2016) Early-life gut microbiome composition and milk allergy resolution. J Allergy Clin Immunol 138:1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, Fiocchi A, Chiang W, Beyer K, Wood R, Hourihane J, Jones SM, Lack G, Sampson HA (2012) ICON: food allergy. J Allergy Clin Immunol 129:906–920. [DOI] [PubMed] [Google Scholar]
- Busquets O, Ettcheto M, Cano A, P RM, Sanchez-Lopez E, Espinosa-Jimenez T, Verdaguer E, Dario Castro-Torres R, Beas-Zarate C, F XS, Olloquequi J, Auladell C, Folch J, Camins A (2019) Role of c-Jun N-terminal kinases (JNKs) in epilepsy and metabolic cognitive impairment. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Holmes SP (2017) Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. Isme j 11:2639–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro S, Frigo AC, Perin M, Stefani S, Cardarelli C, Bozzetto S, Baraldi E, Zanconato S (2012) Impact of oral immunotherapy on quality of life in children with cow milk allergy: a pilot study. Int J Immunopathol Pharmacol 25:793–798. [DOI] [PubMed] [Google Scholar]
- Cenit MC, Sanz Y, Codoner-Franch P (2017) Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol 23:5486–5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Jope RS, Beurel E (2015) A pre-conditioning stress accelerates increases in mouse plasma inflammatory cytokines induced by stress. BMC Neurosci 16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S (2007) Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol 73:7767–7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Dauge V, Naudon L, Rabot S (2014) Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 42:207–217. [DOI] [PubMed] [Google Scholar]
- Cummings AJ, Knibb RC, King RM, Lucas JS (2010) The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy 65:933–945. [DOI] [PubMed] [Google Scholar]
- Davison HM (1949) Cerebral allergy. South Med J 42:712–716. [DOI] [PubMed] [Google Scholar]
- de Theije CG, Wu J, Koelink PJ, Korte-Bouws GA, Borre Y, Kas MJ, Lopes da Silva S, Korte SM, Olivier B, Garssen J, Kraneveld AD (2014) Autistic-like behavioural and neurochemical changes in a mouse model of food allergy. Behav Brain Res 261:265–274. [DOI] [PubMed] [Google Scholar]
- du Toit G, Meyer R, Shah N, Heine RG, Thomson MA, Lack G, Fox AT (2010) Identifying and managing cow’s milk protein allergy. Arch Dis Child Educ Pract Ed 95:134–144. [DOI] [PubMed] [Google Scholar]
- DunnGalvin A, Hourihane JO, Frewer L, Knibb RC, Oude Elberink JN, Klinge I (2006) Incorporating a gender dimension in food allergy research: a review. Allergy 61:1336–1343. [DOI] [PubMed] [Google Scholar]
- Dupont C (2014) Diagnosis of cow’s milk allergy in children: determining the gold standard? Expert Rev Clin Immunol 10:257–267. [DOI] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110:9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro MA, Van Lieshout RJ, Ohayon J, Scott JG (2016) Emotional and behavioral problems in adolescents and young adults with food allergy. Allergy 71:532–540. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, McTeague M, Sandler R, Wexler H, Marlowe EM, Collins MD, Lawson PA, Summanen P, Baysallar M, Tomzynski TJ, Read E, Johnson E, Rolfe R, Nasir P, Shah H, Haake DA, Manning P, Kaul A (2002) Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 35:S6–s16. [DOI] [PubMed] [Google Scholar]
- Fritscher-Ravens A, Pflaum T, Mosinger M, Ruchay Z, Rocken C, Milla PJ, Das M, Bottner M, Wedel T, Schuppan D (2019) Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology 157:109–118 e105. [DOI] [PubMed] [Google Scholar]
- Garg N, Silverberg JI (2014) Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol 112:525–532. [DOI] [PubMed] [Google Scholar]
- Germundson DL, Nagamoto-Combs K (2020) Isotype-specific detection of serum immunoglobulins against allergens. Methods Mol Biol. 2021;2223:159–167. doi: 10.1007/978-1-0716-1001-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germundson DL, Vendsel LP, Nagamoto-Combs K (2020) Region-specific regulation of central histaminergic H3 receptor expression in a mouse model of cow’s milk allergy. Brain Res 1749:147148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germundson DL, Smith NA, Vendsel LP, Kelsch AV, Combs CK, Nagamoto-Combs K (2018) Oral sensitization to whey proteins induces age- and sex-dependent behavioral abnormality and neuroinflammatory responses in a mouse model of food allergy: a potential role of mast cells. J Neuroinflammation 15:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Rodgin S, Goldman R, Rodriguez J, deVos G, Serebrisky D, Feldman JM (2017) Food allergy and anxiety and depression among ethnic minority children and their caregivers. J Pediatr 187:258–264.e251. [DOI] [PubMed] [Google Scholar]
- Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, Joseph J, Gertie JA, Xu L, Collet MA, Grassmann JDS, Simoneau T, Chiang D, Berin MC, Craft JE, Weinstein JS, Williams A, Eisenbarth SC (2019) Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, Nadeau KC (2018) The public health impact ofparent-reported childhood food allergies in the United States. Pediatrics 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, Schleimer RP, Nadeau KC (2019) Prevalence and severity of food allergies among US adults. JAMA Netw Open 2:e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hak E, de Vries TW, Hoekstra PJ, Jick SS (2013) Association of childhood attention-deficit/hyperactivity disorder with atopic diseases and skin infections? A matched case-control study using the General Practice Research Database. Ann Allergy Asthma Immunol 111:102–106.e102. [DOI] [PubMed] [Google Scholar]
- HayGlass KT, Nashed B, Haile S, Marshall AJ, Thomas W (2005) C57Bl/6 and BALB/c mice do not represent default Th1 and Th2 strains in allergen-driven immune responses. J Allergy Clin Immunol 115:S258. doi 10.1016/j.jaci.2004.12.1041. [DOI] [Google Scholar]
- Heaney LG, Conway E, Kelly C, Gamble J (2005) Prevalence of psychiatric morbidity in a difficult asthma population: relationship to asthma outcome. Respir Med 99:1152–1159. [DOI] [PubMed] [Google Scholar]
- Herbert L, Shemesh E, Bender B (2016) Clinical management of psychosocial concerns related to food allergy. J Allergy Clin Immunol Pract 4:205–213. [DOI] [PubMed] [Google Scholar]
- Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R, Payami H (2017) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DJ, Hosking CS (1995) The cow milk allergy complex: overlapping disease profiles in infancy. Eur J Clin Nutr 49 Suppl 1:S1–12. [PubMed] [Google Scholar]
- Hussain M, Bonilla-Rosso G, Kwong Chung CKC, Bäriswyl L, Rodriguez MP, Kim BS, Engel P, Noti M (2019) High dietary fat intake induces a microbiota signature that promotes food allergy. J Allergy Clin Immunol 144:157–170.e158. [DOI] [PubMed] [Google Scholar]
- Inoue R, Sawai T, Sawai C, Nakatani M, Romero-Perez GA, Ozeki M, Nonomura K, Tsukahara T (2017) A preliminary study of gut dysbiosis in children with food allergy. Biosci Biotechnol Biochem 81:2396–2399. [DOI] [PubMed] [Google Scholar]
- Jouvin-Marche E, Morgado MG, Leguern C, Voegtle D, Bonhomme F, Cazenave PA (1989) The mouse Igh-1a and Igh-1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics 29:92–97. [DOI] [PubMed] [Google Scholar]
- Kelly C, Gangur V (2009) Sex disparity in food allergy: Evidence from the PubMed Database. J Allergy (Cairo) 2009:159845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, C OB, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG (2016) Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82:109–118. [DOI] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, Cheon KA, Kim SJ, Kim YK, Lee H, Song DH, Grinker RR (2011) Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry 168:904–912. [DOI] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16:626–638. [DOI] [PubMed] [Google Scholar]
- Knight AK, Blazquez AB, Zhang S, Mayer L, Sampson HA, Berin MC (2007) CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. Am J Physiol Gastrointest Liver Physiol 293:G1234–1243. [DOI] [PubMed] [Google Scholar]
- Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, Mearin ML, Papadopoulou A, Ruemmele FM, Staiano A, Schäppi MG, Vandenplas Y (2012) Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr 55:221–229. [DOI] [PubMed] [Google Scholar]
- Kourosh A, Luna RA, Balderas M, Nance C, Anagnostou A, Devaraj S, Davis CM (2018) Fecal microbiome signatures are different in food-allergic children compared to siblings and healthy children. Pediatr Allergy Immunol 29:545–554. [DOI] [PubMed] [Google Scholar]
- Kulas JA, Hettwer JV, Sohrabi M, Melvin JE, Manocha GD, Puig KL, Gorr MW, Tanwar V, McDonald MP, Wold LE, Combs CK (2018) In utero exposure to fine particulate matter results in an altered neuroimmune phenotype in adult mice. Environ Pollut 241:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont S, Guery JC (2019) Deconstructing the sex bias in allergy and autoimmunity: From sex hormones and beyond. Adv Immunol 142:35–64. [DOI] [PubMed] [Google Scholar]
- Lawrence MG, Woodfolk JA, Schuyler AJ, Stillman LC, Chapman MD, Platts-Mills TA (2017) Half-life of IgE in serum and skin: Consequences for anti-IgE therapy in patients with allergic disease. J Allergy Clin Immunol 139:422–428 e424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Cui L, Yang Y, Miao J, Zhao X, Zhang J, Cui G, Zhang Y (2019) Gut microbiota differs between Parkinson’s disease patients and healthy controls in Northeast China . Front Mol Neurosci 12:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA (1999) A murine model of IgE-mediated cow’s milk hypersensitivity. J Allergy Clin Immunol 103:206–214. [DOI] [PubMed] [Google Scholar]
- Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, Massing M, Cohn RD, Zeldin DC (2010) National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol 126:798–806.e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh W, Tang MLK (2018) The epidemiology of food allergy in the global context. Int J Environ Res Public Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Van de Water J, Ashwood P, Hertz-Picciotto I (2015) Asthma and allergies in children with autism spectrum disorders: Results from the CHARGE study. Autism Res 8:567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeta K, Hattori S, Ikutomo J, Edamatsu H, Bilasy SE, Miyakawa T, Kataoka T (2018) Comprehensive behavioral analysis of mice deficient in Rapgef2 and Rapgef6, a subfamily of guanine nucleotide exchange factors for Rap small GTPases possessing the Ras/Rap-associating domain. Mol Brain 11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makabe-Kobayashi Y, Hori Y, Adachi T, Ishigaki-Suzuki S, Kikuchi Y, Kagaya Y, Shirato K, Nagy A, Ujike A, Takai T, Watanabe T, Ohtsu H (2002) The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J Allergy Clin Immunol 110:298–303. [DOI] [PubMed] [Google Scholar]
- Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A (2016) Gut microbiota in autism and mood disorders. World J Gastroenterol 22:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Martin G, La Rotta Hernandez A, Vazquez de la Torre M, Higaki Y, Zubeldia JM, Baeza ML (2017) Differences in the anaphylactic response between C3H/HeOuJ and BALB/c mice.. Int Arch Allergy Immunol 173:204–212. [DOI] [PubMed] [Google Scholar]
- Markle JG, Fish EN (2014) SeXX matters in immunity. Trends Immunol 35:97–104. [DOI] [PubMed] [Google Scholar]
- McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM (2000) M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164:6166–6173.10843666 [Google Scholar]
- Mostafa GA, Hamza RT, El-Shahawi HH (2008) Allergic manifestations in autistic children: Relation to disease severity. Journal of Pediatric Neurology 6:115–123. [Google Scholar]
- Mousan G, Kamat D (2016) Cow’s milk protein allergy. Clin Pediatr (Phila) 55:1054–1063. [DOI] [PubMed] [Google Scholar]
- Mullins RJ (2007) Paediatric food allergy trends in a community-based specialist allergy practice, 1995–2006. Med J Aust 186:618–621. [DOI] [PubMed] [Google Scholar]
- Murray AL, Booth T, Eisner M, Auyeung B, Murray G, Ribeaud D (2019) Sex differences in ADHD trajectories across childhood and adolescence. Dev Sci 22:e12721. [DOI] [PubMed] [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, Rudi K (2014) Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 26:1155–1162. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Santa K, Yahata T, Sato N, Ohta A, Ohmi Y, Sato T, Hozumi K, Habu S (1997) Involvement of IL-4-producing Vbeta8.2+ CD4+ CD62L- CD45RB- T cells in non-MHC gene-controlled predisposition toward skewing into T helper type-2 immunity in BALB/c mice. J Immunol 158:5698–5706. [PubMed] [Google Scholar]
- Oksaren J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) vegan: Community Ecology Package.
- Pinares-Garcia P, Stratikopoulos M, Zagato A, Loke H, Lee J (2018) Sex: A significant risk factor for neurodevelopmental and neurodegenerative disorders. Brain Sci 8:154. doi: 10.3390/brainsci8080154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (2007) The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164:942–948. [DOI] [PubMed] [Google Scholar]
- Polloni L, Muraro A (2020) Anxiety and food allergy: A review of the last two decades. Clin Exp Allergy 50:420–441. [DOI] [PubMed] [Google Scholar]
- Pulikkan J, Maji A, Dhakan DB, Saxena R, Mohan B, Anto MM, Agarwal N, Grace T, Sharma VK (2018) Gut microbial dysbiosis in Indian children with autism spectrum disorders. Microb Ecol 76:1102–1114. [DOI] [PubMed] [Google Scholar]
- Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, de Vos WM, Satokari R (2015) Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol 81:3655–3662. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CE Jr., Lannigan JA, Kim P, Lee JJ, Fu SM, Sung SS (2010) Murine lung eosinophil activation and chemokine production in allergic airway inflammation. Cell Mol Immunol 7:361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JH, Lee-Sarwar KA, Sordillo J, Bunyavanich S, Zhou Y, O’Connor G, Sandel M, Bacharier LB, Zeiger R, Sodergren E, Weinstock GM, Gold DR, Weiss ST, Litonjua AA (2018) A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy 73:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30:350–358. [DOI] [PubMed] [Google Scholar]
- Seibenhener ML, Wooten MC (2015) Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp:e52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Wang M, Zhang X, He M, Li M, Cheng G, Wan C, He F (2019) Dynamic construction of gut microbiota may influence allergic diseases of infants in Southwest China. BMC Microbiol 19:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicherer SH, Sampson HA (2018) Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol 141:41–58. [DOI] [PubMed] [Google Scholar]
- Smith NA, Nagamoto-Combs K (2020) Induction of hypersensitivity with purified beta-lactoglobulin as a mouse model of cow’s milk allergy. Methods Mol Biol 2223:67–78. doi: 10.1007/978-1-0716-1001-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NA, Germundson DL, Combs CK, Vendsel LP, Nagamoto-Combs K (2019) Astrogliosis associated with behavioral abnormality in a non-anaphylactic mouse model of cow’s milk allergy. Front Cell Neurosci 13:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer F (1954) The allergic tension-fatigue syndrome. Pediatr Clin North Am:1029–1037. [DOI] [PubMed] [Google Scholar]
- Stevens LJ, Kuczek T, Burgess JR, Hurt E, Arnold LE (2010) Dietary sensitivities and ADHD symptoms: Thirty-five years of research. Clinical Pediatrics 50:279–293. [DOI] [PubMed] [Google Scholar]
- Tanda K, Nishi A, Matsuo N, Nakanishi K, Yamasaki N, Sugimoto T, Toyama K, Takao K, Miyakawa T (2009) Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D (2015) Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav 138:179–187. [DOI] [PubMed] [Google Scholar]
- Topal E, Catal F, Soylu N, Ozcan OO, Celiksoy MH, Babayigit A, Erge D, Karakoc HT, Sancak R (2016) Psychiatric disorders and symptoms severity in pre-school children with cow’s milk allergy. Allergol Immunopathol (Madr) 44:445–449. [DOI] [PubMed] [Google Scholar]
- Vieira P, Rajewsky K (1988) The half-lives of serum immunoglobulins in adult mice. Eur J Immunol 18:313–316. [DOI] [PubMed] [Google Scholar]
- Vuong HE, Yano JM, Fung TC, Hsiao EY (2017) The microbiome and host behavior. Annu Rev Neurosci 40:21–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkner M, Warren C, Gupta RS (2015) Quality of life in food allergy patients and their families. Pediatr Clin North Am 62:1453–1461. [DOI] [PubMed] [Google Scholar]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA (2011) Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol 77:6718–6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA (2013) Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism 4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A (2004) Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22:460–466. [DOI] [PubMed] [Google Scholar]
- Wemheuer F, Taylor JA, Daniel R, Johnston E, Meinicke P, Thomas T, Wemheuer B (2020) Tax4Fun2: prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene sequences. Environmental Microbiome 15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DE, Lu J, Langmead B (2019) Improved metagenomic analysis with Kraken 2. Genome Biol 20:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Snetselaar LG, Jing J, Liu B, Strathearn L, Bao W (2018) Association of food allergy and other allergic conditions with autism spectrum disorder in children. JAMA Netw Open 1:e180279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Xu X, Li J, Li F (2019) Association between gut microbiota and autism spectrum disorder: a systematicreview and meta-analysis. Front Psychiatry 10:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghmaie P, Koudelka CW, Simpson EL (2013) Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol 131:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Chen C-L, Yu D, Liu Z (2020) Roles of follicular helper and regulatory T cells in allergic diseases and allergen immunotherapy. Allergy. doi: 10.1111/all.14639. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. Serum levels of BLG-specific immunoglobulin isotypes. Terminal blood samples were used to detect BLG-specific serum IgE (A), IgG1 (B) and IgG2a (C) using ELISA. Optical density values at 450 nm were used to plot the graphs after subtracting the background values at 550 nm (OD450-550). Sham mice (open bars with open triangles); BLG mice (striped bars with filled diamonds); male C57BL/6J (blue); male BALB/cJ (red); female C57BL/6J (green); female BALB/cJ (orange). Bars indicate group average values in OD450-550 ± SEM (n = 10 per group), two-way ANOVA.
S2. Time-dependent changes in the parameter measurements in the open-field activity monitoring. Overall activities of male (S1A) and female (S1B) sham and BLG-sensitized mice in an open-field arena were recorded for 10 min, and the activity parameters indicated in the y-axes were quantified by ANY-maze software. The values were graphed in relation to time in minutes (x-axis) during the test duration. Sham: open triangles; BLG-sensitized: filled diamonds. Values indicate group average ± SEM (n = 8-10). *p < 0.05 when compared to the sham mice at the same time point by t-test.
S3. General locomotor activities recorded during the open-field activity monitoring. Total distance traveled and total time immobile were computed using ANY-maze software to assess potential effects of BLG sensitization on overall activities. Sham mice (open bars with open triangles); BLG mice (striped bars with filled diamonds); male C57BL/6J (blue); male BALB/cJ (red); female C57BL/6J (green) female BALB/cJ (orange). Bars indicate group average values ± SEM (n = 8-10 per group), two-way ANOVA.
S4. Complete cytokine/chemokine array data using Quantibody Mouse Cytokine Array 5.
S5. Extended diversity indices. In addition to the observed species, Chao1, Shannon, and Simpson indices were used to compare the diversity between sex-matched groups.
S6. Complete microbiome profiles of all experimental groups.
S7. Complete KEGG Pathway analysis performed based on the sensitization-induced microbiome changes. Tax4Fun2 reference database was used to identify differential activation of pathways based upon changes in microbiome. Level 1 pathway classifications with an adjusted p-value < 0.05 (t-test with Bonferroni correction) and average log2 greater or less than 1 (fold changes greater or less than 2) are displayed in a heatmap grouped by level 2 classification.