Abstract
Major depressive disorder (MDD) is a common psychiatric illness that manifests in sex-influenced ways. Men and women may experience depression differently and also respond to various antidepressant treatments in sex-influenced ways. Ketamine, which is now being used as a rapid-acting antidepressant, is likely the same. To date, the majority of studies investigating treatment outcomes in MDD do not disaggregate the findings in males and females, and this is also true for ketamine. This review aims to highlight that gap by exploring pre-clinical data—at a behavioral, molecular, and structural level—and recent clinical trials. Sex hormones, particularly estrogen and progesterone, influence the response at all levels examined, and sex is therefore a critical factor to examine when looking at ketamine response. Taken together, the data show females are more sensitive to ketamine than males, and it might be possible to monitor the phase of the menstrual cycle to mitigate some risks associated with the use of ketamine for females with MDD. Based on the studies reviewed in this article, we suggest that ketamine should be administered adhering to sex-specific considerations.
Keywords: BDNF, cytochromes, low-dose ketamine, rapid-acting antidepressants, sexual dimorphism
Introduction
Major depressive disorder (MDD) is a common psychiatric illness and one of the leading causes of years lived with disability worldwide (Collins et al., 2011; James et al., 2018; Vos et al., 2020). The lifetime prevalence of MDD is more than 20% (Harvard Medical School 2007; Hasin et al., 2018), and at its most severe form, MDD can result in suicide (Turecki and Brent 2016; Bachmann 2018). Women are twice as likely to be affected by MDD (Kessler 2003), and evidence suggests that sex can influence response to antidepressant treatments (Khan et al., 2005; Keers and Aitchison 2010), resulting in a major health disparity within the field. Though the majority of antidepressant trials (89%) report the inclusion of male and female participants, less than 1% report an intention to disaggregate by sex (Weinberger et al., 2010), and only a small minority of published papers in psychiatry (16%) use stratified analyses, pointing to a general lack of sex considered in mental health research (Howard et al., 2017) despite evidence of sexual dimorphism in the field (Salk et al., 2017; Eid et al., 2019; Hyde and Mezulis 2020; Kang et al., 2020)
Current first-line pharmacotherapies for MDD include selective serotonin/norepinephrine reuptake inhibitors, which take multiple weeks to show therapeutic effects and only 30%–40% of people respond to the first line of therapeutics (Nierenberg et al., 2000; Lieberman et al., 2008; Machado-Vieira et al., 2010; Al-Harbi 2012; Kato et al., 2018). Given the need for therapeutics with higher efficacy and shorter onset latency, the field has shifted focus to rapid-acting antidepressants such as ketamine.
The effect of a single infusion of ketamine can last 1 to 2 weeks (Berman et al., 2000; Zarate et al., 2006; Price et al., 2009; Diazgranados et al., 2010a, 2010b; Murrough et al., 2013; Grunebaum et al., 2018), and repeated infusions have safely resulted in a cumulative and sustained effect for up to 3 weeks (aan het Rot et al., 2010; Murrough et al., 2013; Shiroma et al., 2014; Cusin et al., 2016; Singh et al., 2016; Phillips et al., 2019). Some of the characteristic features of MDD, including decreased grey matter volume in the prefrontal cortex (PFC) and hippocampus (HC) (Salvadore et al., 2011; MacMaster et al., 2014) as well as decreased plasma and serum levels of brain-derived neurotrophic factor (Lee et al., 2007; Bocchio-Chiavetto et al., 2010; Kishi et al., 2018), are ameliorated by antidepressant treatments (Castrén et al., 2007), including ketamine.
Similar to standard antidepressant therapies, both the positive and negative outcomes of ketamine treatment seem to differ between sexes; therefore, it is imperative to understand the variations to ensure safe and effective treatment. It is important to note that sex refers to the biological differences between males and females, often in connection with reproductive functions, whereas gender is a social construct that has given rise to masculinity and femininity (Short et al., 2013). In this review, we focus on sex differences. This review will discuss the mechanisms of action of ketamine and explore work in preclinical models demonstrating the effects of sex on behavioral responses and molecular, structural, and functional changes in the brain. Finally, we will compare these data with clinical studies and discuss how they relate.
KETAMINE MECHANISM OF ACTION
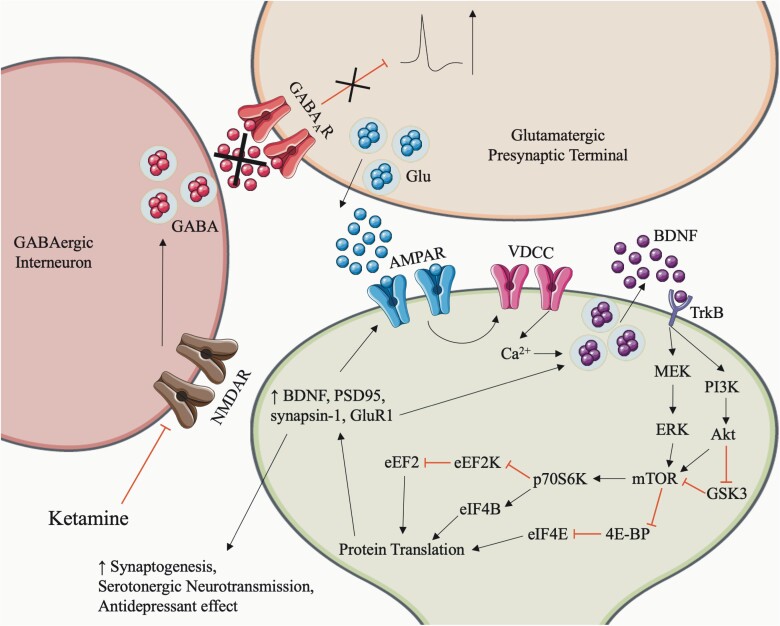
Ketamine acts on a number of cellular processes, including but not limited to blocking NMDA channels, delta and mu-opioid agonism, reduction in cholinergic neuromodulation, and increased release of neurosteroids (Sleigh et al., 2014); however, the following mechanism is the one most associated with its antidepressant effects. Ketamine is an uncompetitive NMDA receptor antagonist (Orser et al., 1997), and its inhibitory action on NMDA receptors is use dependent; specifically, it only blocks open-state receptors on tonically firing GABAergic inhibitory interneurons (MacDonald et al., 1987; Duman et al., 2016). The decreased GABAergic neurotransmission disinhibits excitatory glutamatergic neurons, causing burst releases of glutamate that activate post-synaptic AMPA receptors (Duman et al., 2016; Widman and McMahon 2018). In this instance, AMPA receptor activation leads to an influx of Ca2+ through L-type voltage-gated calcium channels that subsequently induces the release of brain-derived neurotrophic factor (BDNF) into the synapse (Autry et al., 2011; Lepack et al., 2014; Zhou et al., 2014; Yang et al., 2015; Fukumoto et al., 2019) (Figure 1). Conversely, blocking AMPA receptors abolishes the effects on downstream proteins (Maeng et al., 2008; Li et al., 2010; Zhou et al., 2014). Zanos et al. (2016) found that BDNF is elevated only in the HC and not the PFC following ketamine treatment, contrary to other studies, but these discrepancies may be explained by differences in experimental methods.
Figure 1.
Ketamine mechanism of action: ketamine binds open-state NMDA receptors on GABAergic interneurons, which inhibits their firing. Silencing of the interneurons results in disinhibition of excitatory glutamatergic neurons and a burst-release of glutamate. Glutamate binds AMPA receptors on the post-synaptic membrane, leading to calcium influx through L-type voltage-gated calcium channels (VDCC). This influx causes Bdnf release into the synaptic cleft, which binds TrkB, its receptor, on the post-synaptic membrane. Bdnf binding TrkB activates the Mek and PI3K pathways in the post-synaptic neuron, which lead to Gsk3 inhibition, mTOR activation, and protein translation. Ketamine’s antidepressant effect is driven by the resulting synaptogenesis and serotonergic neurotransmission via increased translation of Bdnf, PSD-95, synapsin-1, and GluR1.
Bdnf binds to the postsynaptic TrkB receptor, activating the MAPK and PI3K signaling pathways (Huang and Reichardt 2003; Yang et al., 2015; Ho et al., 2018), leading to phosphorylation of Mek/Erk and Akt, respectively, and the phosphorylation and activation of mTOR (Li et al., 2010; Duman et al., 2016). Importantly, inhibiting any component of this signaling cascade, that is, Erk, Akt, or mTOR, blocks the antidepressant effects of ketamine (Li et al., 2010). Akt can also phosphorylate Gsk3, releasing its inhibition on mTOR; as such, co-administering Gsk inhibitors with ketamine potentiates its effects and lowers the required dose of ketamine (Inoki et al., 2006; Beurel et al., 2011; Kitagishi et al., 2012; Liu et al., 2013; Dossat et al., 2018). Evidence from post-mortem studies supports ketamine’s role in regulating mTOR-dependent translation showing decreased mTOR expression and increased expression of its negative regulator, REDD1 (Jernigan et al., 2011; Ota et al., 2014). Interestingly, independent of ketamine, Bdnf, Erk, and Akt are directly affected by estrogen and progesterone, implying a very important influence of sex on the action of ketamine, which we expand on in a later section.
Activated mTOR inhibits 4E-BP, resulting in the disinhibition of eIF4E (Hoeffer and Klann 2010). Both 4E-BP1 and 4E-BP2 are required for the synaptic and behavioral effects of ketamine (Aguilar-Valles et al., 2020). mTOR also activates p70S6K, in turn, activating eIF4B and inactivating eEF2K (Hoeffer and Klann, 2010; Autry et al., 2011; Monteggia et al., 2013). The reduction of phosphorylation on eEF2 removes the suppression of protein translation (Autry et al., 2011; Monteggia et al., 2013), resulting in increased synaptic strength and spine size (Kopec et al., 2007) through increased translation of PSD-95, synapsin-1, and GluR1 in the post-synaptic terminal (Li et al., 2010, 2011) (Figure 1).
Ketamine’s half-life is 2 to 3 hours (Mion and Villevieille 2013); therefore, the active metabolites and long-term potentiation by AMPA receptor insertion underlie its short-term and sustained antidepressant effects, respectively (Koike et al., 2011; Cornwell et al., 2012). Cytochrome P450 (CYP) enzymes are responsible for the biotransformation of ketamine into its pharmacologically active metabolites: norketamine (NK), hydroxyketamine (HK), hydroxynorketamine (HNK), and dehydronorketamine (DHNK) (Rao et al., 2016). (2R,6R;2S,6S)-HNK may be able to induce an antidepressant response and cause a glutamate burst and AMPA receptor activation, similar to ketamine, independent of NMDA receptor antagonism, though this is controversial at this time (Zanos et al., 2016; Lumsden et al., 2019). (2R,6R)-HNK is more potent than (2S,6S)-HNK—which reflects the relatively greater potency of (R)-ketamine compared with (S)-ketamine—and also lacks ketamine-induced side effects (Zhang et al., 2014; Yang et al., 2015; Zanos et al., 2016). Sex is again implicated in ketamine function because estrogen and progesterone are involved in regulation of CYP enzymes, which will be discussed in more detail in a later section.
Though the most accepted mechanism starts with NMDA receptor inhibition, Zanos et al. (2016) suggest that it is the metabolite (2R,6R)-HNK that is necessary and sufficient for the antidepressant response, independent of NMDA antagonism. Yang et al. (2017) could not replicate the findings of Zanos et al. (2016) in 2 models of depression. Collingridge et al. (2017) caution against disregarding the NMDAR hypothesis, arguing that it remains the strongest proposed mechanism, and Suzuki et al. (2017) suggest that (2R,6R)-HNK does, in fact, inhibit synaptic NMDA receptors, inducing a similar pathway to ketamine, therefore leaving the debate open on the NMDA inhibition-dependent hypothesis.
Beyond the antidepressant effects of ketamine described above (involving increased translation mediated through BDNF and the mTOR pathway), the effects of ketamine result in increased synaptogenesis, spinogenesis, serotonergic neurotransmission, and changes in functional connectivity in/to the PFC and HC. These changes are capable of rescuing the morphological and biochemical abnormalities present in patients with active MDD and result in symptom amelioration (Li et al., 2010, 2011; Gigliucci et al., 2013; Sos et al., 2013; Yamamoto et al., 2013; Nishitani et al., 2014; Thelen et al., 2016; Pham et al., 2017; Moda-Sava et al., 2019) (Figure 1).
SEX DIFFERENCES IN PRECLINICAL MODELS
Detailed tables corresponding to the main findings presented in this section can be found for the behavioral (supplementary Table 1), molecular (supplementary Table 2), and structural supplementary Table 3) data related to ketamine’s effects.
Behavioral Responses
Females are consistently found to be more sensitive to ketamine both in dosage (Carrier and Kabbaj, 2013; Franceschelli et al., 2015; Saland et al., 2016; Sarkar and Kabbaj, 2016; Zanos et al., 2016; Dossat et al., 2018) and magnitude of the behavioral response (Guo et al., 2016; McDougall et al., 2017; Schoepfer et al., 2019), while males have a prolonged response (Franceschelli et al., 2015). Interestingly, ovariectomized female rodents, like males, do not respond to low-dosage treatment (2.5 mg/kg); however, hormone replacement therapy can rescue ketamine sensitivity, suggesting that behavioral responses can fluctuate with female sex hormones (Carrier and Kabbaj 2013; Saland et al., 2016). Although a gonadectomy does not increase behavioral sensitivity to ketamine in males, cyclic administration of progesterone can elicit a response to a typically insufficient dose, suggesting that testosterone does not have a blunting effect, but rather that ovarian hormones enhance the effects (Saland et al., 2016). Proestrus females respond to lower doses of ketamine than males, and although the same is not true in diestrus females, administration of estrogen receptor (ERα/β) agonists can recover this effect (Dossat et al., 2018). Controlling for estrus staging and hormone cycling is a critical factor of ketamine treatment and needs further research, especially because it is overlooked in much of the available literature.
Addiction to ketamine and the likelihood of adverse events manifest in sex-influenced ways. For example, at equivalent doses, negative side effects are more severe in females than in males (McDougall et al., 2017; Schoepfer et al., 2019). In females, 3 weeks of daily ketamine treatment can result in anxiety- and depressive-type behaviors, an effect not found in males (Thelen et al., 2016). On the other hand, repeated doses of ketamine at levels below the threshold for addiction are more likely to be effective in females (Strong et al., 2017; Schoepfer et al., 2019). As described above, lower doses of ketamine are insufficient to elicit an antidepressant response in males; however, low doses (5 mg/kg) administered chronically rather than acutely appear to elicit this response in male rats (Garcia et al., 2008a, 2008b, 2009). Clinically, this may mean repeated low-dose ketamine treatment can have a sustained antidepressant effect in males and carry a lower risk for dependency while acting faster than traditional antidepressants. Although sex hormones can enhance the antidepressant response to ketamine, they also appear to increase susceptibility to addiction (Wright et al., 2017).
Not all the animal data regarding the addictive potential of ketamine can be easily interpreted. For example, some evidence points to only males demonstrating addictive-like behaviors (Schoepfer et al., 2019), whereas other evidence suggests ketamine acts as positive reinforcer for both sexes but that only females are prone to addictive-like behaviors (Guo et al., 2016). Females have been found to demonstrate aversion to ketamine, which has not been seen in males (Strong et al., 2017). The field will need more studies with consistent experimental designs to reliably detect sex differences in risk of addiction to ketamine.
Enantiomerically pure (R)-ketamine is suggested to be a safer alternative to the racemic form of ketamine because it appears to have fewer aversive effects, including being more potent and free of the psychotomimetic side effects and abuse liability (Yang et al., 2015; Zanos et al., 2016). This may present an option for an efficient antidepressant response while mitigating the aversive side effects associated with ketamine.
Both the test and the stress model can influence the outcome of an experiment (supplementary Table 1). Different behavioral tests are used as a proxy for MDD symptoms, and any given study can use 1 of many or a combination of stress paradigms. Many studies use stress-naïve animals (no stress exposure), which are not ideal for representing the effects of ketamine on depression. Inconsistent dose/treatment regimens can also introduce error or noise in the findings, though even studies using the same dose of ketamine have produced different outcomes. Additionally, ovarian hormone levels appear to be critical mediators of the antidepressant response to ketamine, and most studies do not control for estrus staging. The animal used, including the strain of the animal, can have significant impacts on behavioral response. Unsurprisingly, mice and rats do not respond identically, but even the strain of the animal can introduce another layer of complexity. For example, a study using female rats, all on the same dose/treatment regimen, found differences between the Wistar-Kyoto and Wistar strains (Tizabi et al., 2012). Given these factors influencing ketamine response, we must cautiously extrapolate preclinical data to humans.
Molecular Effects
mTOR and Glutamate
Most studies find similar effects of glutamate in both sexes (Sarkar and Kabbaj 2016; Dossat et al., 2018), though there is some evidence to suggest that the glutamate burst, activation of the mTOR pathway, and upregulation of AMPA subunits occur only in the PFC of male mice and no mTOR activation in the HC of either sex (Thelen et al., 2019). Other evidence suggests ketamine may increase synaptic proteins and decrease glutamate and aspartate in the male HC and increase aspartate in the female PFC (Franceschelli et al., 2015; Thelen et al., 2016). Furthermore, subanesthetic doses of ketamine show sex hormone- and regional-specific effects, inducing mTOR activation differentially in males, diestrus females, and proestrus females (Dossat et al., 2018). Presently, the data in the field are too conflicting to draw conclusions on the exact differences in these aspects of ketamine’s molecular response between males and females (supplementary Table 2).
BDNF
In certain behavioral measures, low levels of forebrain Bdnf in female rodents increases sensitivity to depressive-type behaviors after chronic stress, but not males (Autry et al., 2009), and positive treatment response is associated with increased Bdnf in the dorsal HC in females only (Saland et al., 2016). Independent of ketamine, progesterone can induce phosphorylation of Erk and Akt and upregulate Bdnf expression (Kaur et al., 2007). Estrogen can increase Bdnf through binding its ERE-like element (Sohrabji et al., 1995). Following ketamine treatment, males show increased Bdnf in the PFC and HC, whereas for females, changes depend on hormonal status: proestrus females have higher Bdnf levels in the PFC compared with males and diestrus females, whereas the increase is found in the HC of diestrus females (Dossat et al., 2018). Given the enhancing role of ovarian sex hormones on Bdnf signaling, Bdnf may be a key mediator of the enhanced ketamine sensitivity in females.
Cytochromes
CYP enzymes—specifically CYP2A6, CYP2B6, and CYP3A4—are responsible for the biotransformation of ketamine into its active metabolites: NK, HK, HNK, and DHNK (Desta et al., 2012; Rao et al., 2016). CYP2B6 is the major enzyme that mediates N-demethylation to HNK at therapeutic concentrations (Yanagihara et al., 2001; Portmann et al., 2010; Desta et al., 2012). The positive feedback loop regulating ketamine metabolism appears to be mediated, at least in part, by estrogen. Indeed, estrogen, ketamine, and its metabolites work in an additive fashion to induce transcription of CYP2A6, CYP2B6, ERα, and 3 of the 4 AMPA receptor subunits, while ketamine and its metabolites can also bind ERα directly (Ho et al., 2018). Furthermore, significant differences in plasma growth hormone profiles reveal that hepatic expression of cytochrome enzymes is sex influenced in rodents (Waxman and Holloway 2009). These data suggest sex differences in CYP enzymes and their resulting effects on ketamine metabolism.
Pharmacology and Intracellular Signalling
Studies suggest that there may not be sex differences in mTOR phosphorylation following low-dose (neither 2.5 nor 5 mg/kg) ketamine (Carrier and Kabbaj 2013; Zanos et al., 2016) but that increased sensitivity in proestrus females is accompanied by activation of Akt in the PFC and Akt/CaMKIIα in the HC (Dossat et al., 2018). Though comprehensive studies where the pathways are manipulated are necessary to establish a cause and effect relationship, this may suggest that upstream proteins play a role in mediating sex differences. There is also evidence that ketamine induces ∆FosB expression in a sex-influenced manner in the nucleus accumbens (NAc), though the difference may depend on the treatment regimen and the latency between treatment and sacrificing (Strong et al., 2017; Schoepfer et al., 2019).
Though pharmacokinetic profiles are not likely affected by sex hormonal fluctuations, there are noticeable differences between males and females. For example, peak plasma concentrations of ketamine, NK, HNK, and DHNK differ between males and females with respect to both timing and concentration, and because ketamine is not known to undergo local metabolism in the brain, the distribution or permeability of NK into the brain may be greater in females, whereas males may have slower elimination or greater retention of ketamine in the brain. (Zanos et al., 2016; Saland and Kabbaj 2018). These differences reflect the dissimilarities in CYP enzymes and metabolic capacity and may relate to the duration of ketamine’s effects (Franceschelli et al., 2015; Thelen et al., 2019). Using urine metabolites, it was demonstrated that compared with males, females have higher metabolite fluctuations and unique overall metabolic profiles, identifying sex-specific metabolic trajectories (Guo et al., 2016). Contrary to the previous data, 1 study showed no difference in ketamine-induced behavioral or pharmacokinetic profiles between males and females (Chang et al., 2018).
Discrepancies in molecular studies can be attributed to multiple factors. Supplementary Table 2 outlines the main findings of molecular studies in detail. Studies using whole-tissue fractions contain whole-cell information but mask synapse-specific transcripts/proteins, whereas studies using specialized dissections such as synaptoneurosome fractions are specifically enriched for synapse-specific changes of transcripts and proteins (Williams et al., 2009). Because transcripts and proteins are transiently upregulated, differences in testing latency can result in experimental variability. Finally, various stress models induce different baselines before ketamine is administered, meaning some proteins may be altered before treatment. For example, stress induces brain region–specific changes in BDNF signaling. It decreases BDNF in the PFC and HC but increases BDNF in the NAc and basolateral amygdala (Yu and Chen 2011). If the animals are stress naïve, a ceiling effect may be established, preventing further changes to transcript or protein expression; this is likely true with many proteins that have been analyzed across studies.
Structural and Functional Changes
Synaptogenic effects measured by dendritic spine density are the most evidenced structural changes identified in ketamine treatment. In mice, increases were found in male PFC and in female HC, though equivalent increases were not found in female PFC. The increased spine density in female HC appears to be independent of mTOR activation (Li et al., 2010, 2011; Yang et al., 2015; Sarkar and Kabbaj 2016; Thelen et al., 2019). Male rodents with signs of addictive behavior display increased spine density in the nucleus accumbens shell, but not the core, whereas female spine density increases in both the nucleus accumbens shell and nucleus accumbens core (Strong et al., 2017).
Ketamine treatment leads to increased functional connectivity to the dorsolateral PFC from several subcortical and cortical regions, and functional brain networks associated with emotional regulation, cognitive control, and motivation have been found to be hyperconnected following ketamine treatment (Gopinath et al., 2016). Systemically, both acute and chronic ketamine administration increase body weight and can reverse elevated adrenal weight resulting from chronic mild stress. Supplementary Table 3 outlines the main findings of structural and functional studies in detail.
HUMAN DATA
Clinical trials of ketamine for MDD and treatment-resistant depression (TRD) are still in their infancy, with surprisingly few studies that examine sex differences. In this section, we will discuss the human correlates to preclinical data.
Neuromolecular changes resulting from ketamine treatment are rare in human trials given most protein changes can only be examined directly in brain tissue and cannot be detected in peripheral tissue. Although ketamine is a relatively new treatment for MDD/TRD and data are limited, it has been demonstrated that following ketamine administration, plasma BDNF is elevated at 2 and 24 hours, showing a significant sex effect in which women have higher plasma BDNF at baseline (Woelfer et al., 2019). Post-mortem brain tissue analyses revealed that BDNF levels are reduced in the PFC and HC of female and male depressed suicides, respectively (Hayley et al., 2015).
Changes in functional connectivity from ketamine treatment have also been described. Patients with MDD have lower global brain connectivity, but 24 hours after receiving ketamine, increased global brain connectivity can be detected in the PFC. These increases are specifically associated with treatment response and show evidence of synaptogenesis (Abdallah et al., 2017). In both humans and rats, ketamine induces a robust increase in PFC-HC coupling (Grimm et al., 2015). Progesterone alone can increase functional connectivity from both bilateral dorsolateral PFC and bilateral sensorimotor cortices with the HC (Arélin et al., 2015) that fluctuate throughout the menstrual cycle. Ketamine increases activity in the midcingulate, dorsal anterior cingulate cortex, insula, and thalamus and decreases activity in the subgenual/subcallosal anterior cingulate cortex, orbitofrontal cortex, and gyrus rectus (Höflich et al., 2017). The subgenual cortex is thought to be metabolically overactive in TRD (Mayberg et al., 2005), and decreases in orbitofrontal cortex and subgenual activity may predict the dissociative effects of ketamine (Deakin et al., 2008); therefore, it is possible that the cause of the dissociative side effects may also contribute to the antidepressant effects. Ketamine dependency is associated with dose-dependent white matter deficits in the bilateral frontal and left temporoparietal cortices. Because patients with schizophrenia show similar deficits, it is thought that white matter contributes to ketamine’s psychotomimetic side effects (Liao et al., 2010).
Although there do not seem to be significant differences in ketamine treatment response between men and women or between pre- and post-menopausal women, men and women do experience ketamine treatment differently (Coyle and Laws, 2015; Freeman et al., 2019), a fact that may be related to the dose administered. For example, with a 0.5-mg/kg dose of ketamine, women presented higher scores on the Hamilton Depression Rating Scale than men at 24 hours, but when given 1.0 mg/kg of ketamine, women had lower Hamilton Depression Rating Scale scores after 24 hours (Freeman et al., 2019). Moreover, side effects differ between sexes, with men reporting more depersonalization, amnesic, verbal learning deficits, subjective memory loss, and psychotic disorders (Morgan et al., 2006; Zhang et al., 2013; Derntl et al., 2019) and women more likely to report increased nausea, headaches, and cognitive impairment disorders (Zhang et al., 2013; Freeman et al., 2019). In chronic ketamine users, women report more severe withdrawal symptoms such as anxiety, dysphoria, tremors, cognitive impairment, and urinary discomfort (Chen et al., 2014). In addition, although transient hypertension is common with ketamine treatment (aan het Rot et al., 2010; Murrough et al., 2013; Liebe et al., 2017), women reach max diastolic blood pressure faster and more severely than men, with changes almost twofold higher (Liebe et al., 2017). Liebe et al. (2017) suggest extra attention be paid to women with baseline hypertension because of the increased risk of hypertensive crisis (Liebe et al., 2017). Finally, ketamine has greater effects on cardiac output and pain indices (analgesia) in men, whereas women have faster clearance of the drug (Sigtermans et al., 2009). Similar to rodents, these effects may reflect differences in CYP enzymes.
CYP enzymes show sex-influenced expression in humans as well. CYP2A6, CYP2B6, and CYP3A4 expression are all induced by estrogen and progesterone (Higashi et al., 2007; Koh et al., 2012; Choi et al., 2013). CYP2B6 and CYP3A4 are the primary enzymes responsible for the biotransformation of ketamine into NK and HNK in human liver microsomes (Yanagihara et al., 2001; Hijazi and Boulieu 2002). Compared with men, CYP3A4 shows higher expression and activity in women (Hunt et al., 1992; Wolbold et al., 2003; Parkinson et al., 2004). CYP enzymes can help explain some sex differences, including the influence of different metabolic profiles on clinical outcomes. Women have higher DHNK, HNK4a, and HNK4c levels than males—all catalyzed mainly by CYP2B6; males have higher HK5a—catalyzed by CYP3A4/CYP2A6 (Zarate et al., 2012). This is clinically relevant because higher DHNK, HNK4c, and HNK4f levels are associated with lower scores on the Brief Psychiatric Rating Scale and Clinician Administered Dissociative States Scale (Zarate et al., 2012), in line with men having more psychotomimetic and dissociative side effects.
FUTURE DIRECTIONS
Males and females seem to differ in response to ketamine—as they do with other antidepressant therapies—and it is important that these sex-influenced responses be considered when going forward with clinical trials and potential therapeutic regimens of ketamine for MDD/TRD. Preclinical models reveal that females are more sensitive and respond to lower doses of ketamine, likely due to ovarian hormones and different metabolic profiles. There are differences with respect to behavioral, molecular, structural, and functional responses to ketamine in preclinical models, and future clinical research needs to include more women and closely examine the differences between sexes. Given that ovarian hormones have a significant influence on pharmacodynamics and metabolism, the phase of the menstrual cycle should be taken into account. Additionally, studies need to determine long-term safety and efficacy in both sexes. As seen in preclinical studies, ketamine doses that are typically insufficient acutely may be effective as a chronic regimen (including in males), which needs to be followed-up in humans. To date, the acute effects of ketamine are likely similar in both sexes, though side effects vary. As such, males should be monitored more closely for psychiatric symptoms such as dissociation and psychosis, whereas physical symptoms such as hypertension and nausea should be particularly monitored in females.
CONCLUSION
Based on the preclinical and clinical data, it seems that long-term sustained use of low-dose ketamine cannot be administered equivalently between sexes, given the risk for misuse and side effect profiles. In addition, the use of enantiomerically pure (R)-ketamine may mitigate risk, because preclinical data suggest it is more potent, could potentially be given at a lower dose, and may be free of the psychotomimetic side effects of its racemic form. Both men and women experience adverse events following ketamine administration; however, they are mostly transient and commonly do not pose significant health risks to the patient. When possible, for women, administration of ketamine near the onset of the follicular phase—when estrogen and progesterone levels are at their lowest—may also reduce risk because ovarian hormones likely increase susceptibility to adverse events and addiction. Overall, based on the studies reviewed in this article, we suggest that ketamine should be administered in a sex-specific manner.
Supplementary Material
Acknowledgments
We acknowledge the funding that supported this work. G.T. holds a Canada Research Chair (Tier 1) and is supported by grants from the Canadian Institute of Health Research (CIHR) (FDN148374 and ENP161427 [ERA-NET ERA PerMed]). C.N is funded by the Réseau québécois sur le suicide, les troubles de l’humeur et les troubles associés (RQSHA), Adversity and Mental Health (AMH) Collaborative Initiative, and McGill-Douglas Max Planck Institute of Psychiatry.
Interest Statement
None.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145. [DOI] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, DeWilde KE, Wong E, Anticevic A, Tang CY, Iosifescu DV, Charney DS, Murrough JW (2017) Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 42:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Valles A, De Gregorio D, Matta-Camacho E, Eslamizade MJ, Khlaifia A, Skaleka A, Lopez-Canul M, Torres-Berrio A, Bermudez S, Rurak GM, Simard S, Salmaso N, Gobbi G, Lacaille JC, Sonenberg N (2020) Antidepressant actions of ketamine engage cell-specific translation via eIF4E. Nature 590:315–319. [DOI] [PubMed] [Google Scholar]
- Al-Harbi KS (2012) Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence 6:369–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arélin K, Mueller K, Barth C, Rekkas PV, Kratzsch J, Burmann I, Villringer A, Sacher J (2015) Progesterone mediates brain functional connectivity changes during the menstrual cycle-a pilot resting state MRI study. Front Neurosci 9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Cheng P, Monteggia LM (2009) Gender-specific impact of brain-derived neurotrophic factor signaling on stress-induced depression-like behavior. Biol Psychiatry 66:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann S (2018) Epidemiology of suicide and the psychiatric perspective. Int J Environ Res Public Health 15:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Beurel E, Song L, Jope RS (2011) Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry 16:1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Nielsen MG, Placentino A, Giovannini C, Rillosi L, Ventriglia M, Riva MA, Gennarelli M (2010) Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry 11:763–773. [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M (2013) Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70:27–34. [DOI] [PubMed] [Google Scholar]
- Castrén E, Võikar V, Rantamäki T (2007) Role of neurotrophic factors in depression. Curr Opin Pharmacol 7:18–21. [DOI] [PubMed] [Google Scholar]
- Chang L, Toki H, Qu Y, Fujita Y, Mizuno-Yasuhira A, Yamaguchi JI, Chaki S, Hashimoto K (2018) No sex-specific differences in the acute antidepressant actions of (R)-ketamine in an inflammation model. Int J Neuropsychopharmacol 21: 932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Huang MC, Lin SK (2014) Gender differences in subjective discontinuation symptoms associated with ketamine use. Subst Abuse Treat Prev Policy 9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Koh KH, Jeong H (2013) Isoform-specific regulation of cytochromes P450 expression by estradiol and progesterone. Drug Metab Dispos 41:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Lee Y, Bortolotto ZA, Kang H, Lodge D (2017) Antidepressant actions of ketamine versus hydroxynorketamine. Biol Psychiatry 81:e65–e67. [DOI] [PubMed] [Google Scholar]
- Collins PY, et al. ; Scientific Advisory Board and the Executive Committee of the Grand Challenges on Global Mental Health (2011) Grand challenges in global mental health. Nature 475:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA Jr (2012) Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry 72:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CM, Laws KR (2015) The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol 30:152–163. [DOI] [PubMed] [Google Scholar]
- Cusin C, Ionescu DF, Pavone KJ, Akeju O, Cassano P, Taylor N, Eikermann M, Durham K, Swee MB, Chang T, Dording C, Soskin D, Kelley J, Mischoulon D, Brown EN, Fava M (2016) Ketamine augmentation for outpatients with treatment-resistant depression: preliminary evidence for two-step intravenous dose escalation. Aust N Z J Psychiatry 51:55–64. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM (2008) Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry 65:154–164. [DOI] [PubMed] [Google Scholar]
- Derntl B, Hornung J, Sen ZD, Colic L, Li M, Walter M (2019) Interaction of sex and age on the dissociative effects of ketamine action in young healthy participants. Front Neurosci 13:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desta Z, Moaddel R, Ogburn ET, Xu C, Ramamoorthy A, Venkata SL, Sanghvi M, Goldberg ME, Torjman MC, Wainer IW (2012) Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica 42:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA Jr (2010a) Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 71:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr (2010b) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat AM, Wright KN, Strong CE, Kabbaj M (2018) Behavioral and biochemical sensitivity to low doses of ketamine: influence of estrous cycle in C57BL/6 mice. Neuropharmacology 130:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid RS, Gobinath AR, Galea LAM (2019) Sex differences in depression: insights from clinical and preclinical studies. Prog Neurobiol 176:86–102. [DOI] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM (2015) Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience 290:49–60. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Papakostas GI, Hoeppner B, Mazzone E, Judge H, Cusin C, Mathew S, Sanacora G, Iosifescu D, DeBattista C, Trivedi MH, Fava M (2019) Sex differences in response to ketamine as a rapidly acting intervention for treatment resistant depression. J Psychiatr Res 110:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Fogaça MV, Liu RJ, Duman C, Kato T, Li XY, Duman RS (2019) Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci U S A 116:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008a) Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels. Basic Clin Pharmacol Toxicol 103:502–506. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008b) Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 32:140–144. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, Gavioli EC, Quevedo J (2009) Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry 33:450–455. [DOI] [PubMed] [Google Scholar]
- Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A (2013) Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl) 228:157–166. [DOI] [PubMed] [Google Scholar]
- Gopinath K, Maltbie E, Urushino N, Kempf D, Howell L (2016) Ketamine-induced changes in connectivity of functional brain networks in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology (Berl) 233:3673–3684. [DOI] [PubMed] [Google Scholar]
- Grimm O, Gass N, Weber-Fahr W, Sartorius A, Schenker E, Spedding M, Risterucci C, Schweiger JI, Böhringer A, Zang Z, Tost H, Schwarz AJ, Meyer-Lindenberg A (2015) Acute ketamine challenge increases resting state prefrontal-hippocampal connectivity in both humans and rats. Psychopharmacology (Berl) 232:4231–4241. [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Galfalvy HC, Choo TH, Keilp JG, Moitra VK, Parris MS, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ (2018) Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry 175:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Tang Q, Ye Y, Lu X, Chen F, Dai X, Yan Y, Liao L (2016) Effects of gender on ketamine-induced conditioned placed preference and urine metabonomics. Regul Toxicol Pharmacol 77:263–274. [DOI] [PubMed] [Google Scholar]
- Harvard Medical School (2007) National Comorbidity Survey. Available at: https://www.hcp.med.harvard.edu/ncs/index.php. Data Table 1: https://www.hcp.med.harvard.edu/ncs/ftpdir/table_ncsr_LTprevgenderxage.pdf. Accessed November 24, 2020.
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, Grant BF (2018) Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 75:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Du L, Litteljohn D, Palkovits M, Faludi G, Merali Z, Poulter MO, Anisman H (2015) Gender and brain regions specific differences in brain derived neurotrophic factor protein levels of depressed individuals who died through suicide. Neurosci Lett 600:12–16. [DOI] [PubMed] [Google Scholar]
- Higashi E, Fukami T, Itoh M, Kyo S, Inoue M, Yokoi T, Nakajima M (2007) Human CYP2A6 is induced by estrogen via estrogen receptor. Drug Metab Dispos 35:1935–1941. [DOI] [PubMed] [Google Scholar]
- Hijazi Y, Boulieu R (2002) Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos 30:853–858. [DOI] [PubMed] [Google Scholar]
- Ho MF, Correia C, Ingle JN, Kaddurah-Daouk R, Wang L, Kaufmann SH, Weinshilboum RM (2018) Ketamine and ketamine metabolites as novel estrogen receptor ligands: induction of cytochrome P450 and AMPA glutamate receptor gene expression. Biochem Pharmacol 152:279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E (2010) mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höflich A, Hahn A, Küblböck M, Kranz GS, Vanicek T, Ganger S, Spies M, Windischberger C, Kasper S, Winkler D, Lanzenberger R (2017) Ketamine-dependent neuronal activation in healthy volunteers. Brain Struct Funct 222:1533–1542. [DOI] [PubMed] [Google Scholar]
- Howard LM, Ehrlich AM, Gamlen F, Oram S (2017) Gender-neutral mental health research is sex and gender biased. Lancet Psychiatry 4:9–11. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642. [DOI] [PubMed] [Google Scholar]
- Hunt CM, Westerkam WR, Stave GM (1992) Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol 44:275–283. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH (2020) Gender differences in depression: biological, affective, cognitive, and sociocultural factors. Harv Rev Psychiatry 28:4–13. [DOI] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126:955–968. [DOI] [PubMed] [Google Scholar]
- James SL et al. (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B (2011) The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 35:1774–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Park Y, Yoo KH, Kim KT, Kim ES, Kim JW, Kim SW, Shin IS, Yoon JS, Kim JH, Kim JM (2020) Sex differences in the genetic architecture of depression. Sci Rep 10:9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, et al. ; SUN☺D Investigators . (2018) Optimising first- and second-line treatment strategies for untreated major depressive disorder - the SUN☺D study: a pragmatic, multi-centre, assessor-blinded randomised controlled trial. BMC Med 16:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M (2007) Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res 85:2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keers R, Aitchison KJ (2010) Gender differences in antidepressant drug response. Int Rev Psychiatry 22:485–500. [DOI] [PubMed] [Google Scholar]
- Kessler RC (2003) Epidemiology of women and depression. J Affect Disord 74:5–13. [DOI] [PubMed] [Google Scholar]
- Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA (2005) Sex differences in antidepressant response in recent antidepressant clinical trials. J Clin Psychopharmacol 25:318–324. [DOI] [PubMed] [Google Scholar]
- Kishi T, Yoshimura R, Ikuta T, Iwata N (2018) Brain-derived neurotrophic factor and major depressive disorder: evidence from meta-analyses. Front Psychiatry 8:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagishi Y, Kobayashi M, Kikuta K, Matsuda S (2012) Roles of PI3K/AKT/GSK3/mTOR pathway in cell signaling of mental illnesses. Depress Res Treat 2012:752563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KH, Jurkovic S, Yang K, Choi SY, Jung JW, Kim KP, Zhang W, Jeong H (2012) Estradiol induces cytochrome P450 2B6 expression at high concentrations: implication in estrogen-mediated gene regulation in pregnancy. Biochem Pharmacol 84:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S (2011) Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 224:107–111. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Real E, Kessels HW, Malinow R (2007) GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci 27:13706–13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Kim H, Park SH, Kim YK (2007) Decreased plasma BDNF level in depressive patients. J Affect Disord 101:239–244. [DOI] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS (2014) BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X, Liu T, Chen X, Fletcher PC, Hao W (2010) Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain 133:2115–2122. [DOI] [PubMed] [Google Scholar]
- Liebe T, Li S, Lord A, Colic L, Krause AL, Batra A, Kretzschmar MA, Sweeney-Reed CM, Behnisch G, Schott BH, Walter M (2017) Factors influencing the cardiovascular response to subanesthetic ketamine: a randomized, placebo-controlled trial. Int J Neuropsychopharmacol 20:909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DZ, Montgomery SA, Tourian KA, Brisard C, Rosas G, Padmanabhan K, Germain J- M, Pitrosky B (2008) A pooled analysis of two placebo-controlled trials of desvenlafaxine in major depressive disorder. Intl Clin Psychopharmacol 23:188–197. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK (2013) GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology 38:2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden EW, et al. (2019) Antidepressant-relevant concentrations of the ketamine metabolite (2R,6R)-hydroxynorketamine do not block NMDA receptor function. Proc Natl Acad Sci U S A 116:5160–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JF, Miljkovic Z, Pennefather P (1987) Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol 58:251–266. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Baumann J, Wheeler-Castillo C, Latov D, Henter ID, Salvadore G, Zarate CA (2010) The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmaceuticals 3:19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster FP, Carrey N, Langevin LM, Jaworska N, Crawford S (2014) Disorder-specific volumetric brain difference in adolescent major depressive disorder and bipolar depression. Brain Imaging Behav 8:119–127. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45:651–660. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Moran AE, Baum TJ, Apodaca MG, Real V (2017) Effects of ketamine on the unconditioned and conditioned locomotor activity of preadolescent and adolescent rats: impact of age, sex, and drug dose. Psychopharmacology 234:2683–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion G, Villevieille T (2013) Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther 19:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, Witztum J, Shaver DC, Rosenthal DL, Alway EJ, Lopez K, Meng Y, Nellissen L, Grosenick L, Milner TA, Deisseroth K, Bito H, Kasai H, Liston C (2019) Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 364:eaat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Gideons E, Kavalali ET (2013) The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry 73:1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Perry EB, Cho HS, Krystal JH, D’Souza DC (2006) Greater vulnerability to the amnestic effects of ketamine in males. Psychopharmacology 187:405–414. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg AA, Farabaugh AH, Alpert JE, Gordon J, Worthington JJ, Rosenbaum JF, Fava M (2000) Timing of onset of antidepressant response with fluoxetine treatment. Am J Psychiatry 157:1423–1428. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Nagayasu K, Asaoka N, Yamashiro M, Shirakawa H, Nakagawa T, Kaneko S (2014) Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex. Int J Neuropsychopharmacol 17:1321–1326. [DOI] [PubMed] [Google Scholar]
- Orser BA, Pennefather PS, MacDonald JF (1997) Multiple mechanisms of ketamine blockade of N-methyl-D-aspartate receptors. Anesthesiology 86:903–917. [DOI] [PubMed] [Google Scholar]
- Ota KT, Liu RJ, Voleti B, Maldonado-Aviles JG, Duric V, Iwata M, Dutheil S, Duman C, Boikess S, Lewis DA, Stockmeier CA, DiLeone RJ, Rex C, Aghajanian GK, Duman RS (2014) REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med 20:531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson A, Mudra DR, Johnson C, Dwyer A, Carroll KM (2004) The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol 199:193–209. [DOI] [PubMed] [Google Scholar]
- Pham TH, Mendez-David I, Defaix C, Guiard BP, Tritschler L, David DJ, Gardier AM (2017) Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacology 112:198–209. [DOI] [PubMed] [Google Scholar]
- Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, Owoeye O, Batten LA, Blier P (2019) Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry 176:401–409. [DOI] [PubMed] [Google Scholar]
- Portmann S, Kwan HY, Theurillat R, Schmitz A, Mevissen M, Thormann W (2010) Enantioselective capillary electrophoresis for identification and characterization of human cytochrome P450 enzymes which metabolize ketamine and norketamine in vitro. J Chromatogr A 1217:7942–7948. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao LK, Flaker AM, Friedel CC, Kharasch ED (2016) Role of cytochrome P4502B6 polymorphisms in ketamine metabolism and clearance. Anesthesiology 125:1103–1112. [DOI] [PubMed] [Google Scholar]
- Saland SK, Kabbaj M (2018) Sex differences in the pharmacokinetics of low-dose ketamine in plasma and brain of male and female rats. J Pharmacol Exp Ther 367:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saland SK, Schoepfer KJ, Kabbaj M (2016) Hedonic sensitivity to low-dose ketamine is modulated by gonadal hormones in a sex-dependent manner. Sci Rep 6:21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk RH, Hyde JS, Abramson LY (2017) Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull 143:783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Nugent AC, Lemaitre H, Luckenbaugh DA, Tinsley R, Cannon DM, Neumeister A, Zarate CA Jr, Drevets WC (2011) Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. Neuroimage 54:2643–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Kabbaj M (2016) Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol Psychiatry 80:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer KJ, Strong CE, Saland SK, Wright KN, Kabbaj M (2019) Sex- and dose-dependent abuse liability of repeated subanesthetic ketamine in rats. Physiol Behav 203:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, Lim KO (2014) Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord 155:123–129. [DOI] [PubMed] [Google Scholar]
- Short SE, Yang YC, Jenkins TM (2013) Sex, gender, genetics, and health. Am J Public Health 103 Suppl 1:S93–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigtermans M, Dahan A, Mooren R, Bauer M, Kest B, Sarton E, Olofsen E (2009) S(+)-ketamine effect on experimental pain and cardiac output: a population pharmacokinetic-pharmacodynamic modeling study in healthy volunteers. Anesthesiology 111:892–903. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L (2016) A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry 173:816–826. [DOI] [PubMed] [Google Scholar]
- Sleigh J, Harvey M, Voss L, Denny B (2014) Ketamine – more mechanisms of action than just NMDA blockade. Trends Anaesth Crit Care 4:76–81. [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD (1995) Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A 92:11110–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T (2013) Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol Lett 34:287–293. [PubMed] [Google Scholar]
- Strong CE, Schoepfer KJ, Dossat AM, Saland SK, Wright KN, Kabbaj M (2017) Locomotor sensitization to intermittent ketamine administration is associated with nucleus accumbens plasticity in male and female rats. Neuropharmacology 121:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM (2017) Effects of a ketamine metabolite on synaptic NMDAR function. Nature 546:E1–E3. [DOI] [PubMed] [Google Scholar]
- Thelen C, Sens J, Mauch J, Pandit R, Pitychoutis PM (2016) Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav Brain Res 312:305–312. [DOI] [PubMed] [Google Scholar]
- Thelen C, Flaherty E, Saurine J, Sens J, Mohamed S, Pitychoutis PM (2019) Sex differences in the temporal neuromolecular and synaptogenic effects of the rapid-acting antidepressant drug ketamine in the mouse brain. Neuroscience 398:182–192. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L (2012) Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience 213:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecki G, Brent DA (2016) Suicide and suicidal behaviour. Lancet 387:1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T et al. (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396:1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, Holloway MG (2009) Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol 76:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, McKee SA, Mazure CM (2010) Inclusion of women and gender-specific analyses in randomized clinical trials of treatments for depression. J Womens Health 19:1727–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widman AJ, McMahon LL (2018) Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci U S A 115:E3007–E3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Mehrian Shai R, Wu Y, Hsu YH, Sitzer T, Spann B, McCleary C, Mo Y, Miller CA (2009) Transcriptome analysis of synaptoneurosomes identifies neuroplasticity genes overexpressed in incipient Alzheimer’s disease. PLoS One 4:e4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelfer M, Li M, Colic L, Liebe T, Di X, Biswal B, Murrough J, Lessmann V, Brigadski T, Walter M (2019) Ketamine-induced changes in plasma brain-derived neurotrophic factor (BDNF) levels are associated with the resting-state functional connectivity of the prefrontal cortex. World J Biol Psychiatry 21:696–710. [DOI] [PubMed] [Google Scholar]
- Wolbold R, Klein K, Burk O, Nüssler AK, Neuhaus P, Eichelbaum M, Schwab M, Zanger UM (2003) Sex is a major determinant of CYP3A4 expression in human liver. Hepatology 38:978–988. [DOI] [PubMed] [Google Scholar]
- Wright KN, Strong CE, Addonizio MN, Brownstein NC, Kabbaj M (2017) Reinforcing properties of an intermittent, low dose of ketamine in rats: effects of sex and cycle. Psychopharmacology (Berl) 234:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Ohba H, Nishiyama S, Harada N, Kakiuchi T, Tsukada H, Domino EF (2013) Subanesthetic doses of ketamine transiently decrease serotonin transporter activity: a PET study in conscious monkeys. Neuropsychopharmacology 38:2666–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara Y, Kariya S, Ohtani M, Uchino K, Aoyama T, Yamamura Y, Iga T (2001) Involvement of CYP2B6 in n-demethylation of ketamine in human liver microsomes. Drug Metab Dispos 29:887–890. [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (2015) R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K (2017) (R)-Ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol Psychiatry 82:e43–e44. [DOI] [PubMed] [Google Scholar]
- Yu H, Chen ZY (2011) The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin 32:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, Wainer IW (2012) Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry 72:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu C, Zhang J, Hu L, Song H, Li J, Kang L (2013) Gender differences in abusers of amphetamine-type stimulants and ketamine in southwestern China. Addict Behav 38:1424–1430. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K (2014) R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116:137–141. [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ (2014) Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 29:419–423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.