Abstract
The evolutionarily conserved cohesin complex is required for the establishment and maintenance of sister chromatid cohesion, in turn essential for proper chromosome segregation. RAD21/SCC1 is a regulatory subunit of the mitotic cohesin complex, as it links together all other subunits of the complex. The destruction of RAD21/SCC1 along chromosomal arms and later at centromeres results in the dissociation of the cohesin complex, facilitating chromosome segregation. Here, we report for the first time that mammalian RAD21/SCC1 associates with the axial/lateral elements of the synaptonemal complex along chromosome arms and on centromeres of mouse spermatocytes. Importantly, RAD21/SCC1 is lost from chromosome arms in late prophase I but persists on centromeres. The loss of centromeric RAD21/SCC1 coincides with the separation of sister chromatids at anaphase II. These findings support a role for mammalian RAD21/SCC1 in maintaining sister chromatid cohesion in meiosis.
Keywords: cohesin, sister chromatid cohesion, meiosis, chromosome segregation
Introduction
Accurate chromosome segregation during eukaryotic cell division depends on the establishment and proper maintenance of sister chromatid cohesion (SCC). This function is performed by the evolutionarily conserved cohesin complex. In mitosis of budding yeast, the cohesin complex consists of two SMC (structural maintenance of chromosomes) subunits, SMC1 and SMC3, and two non-SMC subunits, SCC1 and SCC3 (reviewed in Nasmyth, 2001; Jessberger, 2002). SCC1 is a vital component of the complex, and its cleavage by the anaphase effector protease, separase, at the metaphase-to-anaphase transition regulates the disassociation of sister chromatids, thereby allowing their segregation to opposite poles of the spindle (Uhlmann et al, 1999). Thus, SCC1 is central to the structural integrity of the mitotic cohesin complex.
In meiosis, chromosomes segregate in two successive divisions without an intervening DNA replication. During meiosis I (MI), the release of cohesion from between the arms and distal to chiasmata but not at the centromeric regions of sister chromatids results in the ‘reductional' segregation of homologous chromosomes. The two sister chromatids of each homologue remain attached at their centromeres until the ‘equational' division of meiosis II (MII), when centromeric cohesion is finally lost. This pattern of chromosome cohesion loss results in the equal partition of sister chromatids to each haploid gamete.
Although the SCC1 subunit of the cohesin complex is vital for SCC in mitosis, its role in meiosis remains unknown. A paralogous protein, REC8, has an important role in regulating meiotic chromosome segregation in yeast (Klein et al, 1999; Watanabe & Nurse, 1999). However, SCC1-73 mutants with mitotic defects have been shown to affect the efficiency of meiosis, suggesting that SCC1 may have a role in budding yeast meiosis (Klein et al, 1999). In fission yeast, both REC8 and RAD21 (an SCC1 homologue) exist on chromosome cores during MI (Watanabe & Nurse, 1999). However, in both budding and fission yeast, REC8, rather than RAD21/SCC1, is essential for the centromeric cohesion of sister chromatids (Klein et al, 1999; Watanabe & Nurse, 1999; Yokobayashi et al, 2003).
The involvement of RAD21/SCC1 in mammalian meiosis is poorly defined. A detailed study of this important cohesin could further facilitate our understanding of SCC and chromosome segregation during mammalian meiosis. In this study, we analysed the chromosomal distribution of mammalian RAD21/SCC1 during meiosis. Our data provide the first evidence that mammalian RAD21/SCC1 may have an important role in regulating chromosome cohesion and segregation during meiosis.
Results And Discussion
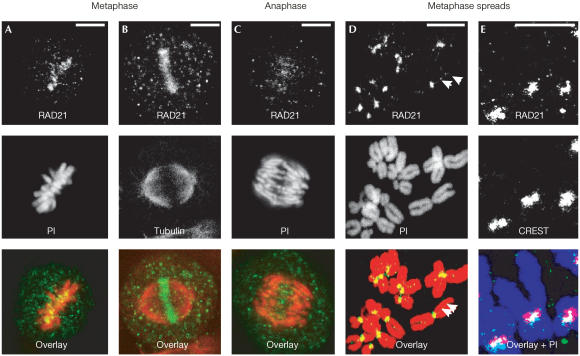
We previously identified a mammalian orthologue of the mitotic cohesin RAD21 (then called hHR21) (McKay et al, 1996). To further characterize the expression of RAD21/SCC1, we generated two independent antibodies against human RAD21/SCC1 (supplementary information online). Both antibodies recognized a protein band of approximately 120 kDa, corresponding to that reported for RAD21/SCC1 on western blots and immunoprecipitation analyses (supplementary Figs 1 and 2 online). Examination of HeLa cells by immunofluorescence revealed that both antibodies stained patchy areas on metaphase-like chromosomes in metaphase-arrested cells (Fig 1D and supplementary Fig 3E online). Such patchy staining was observed on chromosomes along the metaphase plate (Fig 1A,B). Fig 1D clearly demonstrates that these patchy areas of staining corresponded to centromeres on metaphase chromosome spreads. Co-immunostaining with human CREST sera showed that the signal localized between two sister centromeres (Fig 1E), resembling that of SCC1–Myc (Hauf et al, 2001). Thus, we concluded that both antibodies are specific to RAD21/SCC1.
Figure 1.
Immunostaining of RAD21/SCC1 antibodies in HeLa cells. Images were obtained using RAD21 Ab2. Chromosomes were counterstained using propidium iodide (PI). (A) Metaphase showing RAD21/SCC1 (green) and chromosomes (red), (B) co-labelling of RAD21/SCC1 (green) with tubulin (red), (C) anaphase, (D) metaphase chromosome spreads (note the residual RAD21/SCC1 staining between sister chromatids) (arrowheads) and (E) co-labelling of RAD21/SCC1 (green) with CREST-6 sera for centromeres (red) on metaphase chromosome spreads (purple). Scale bar, 10 μm.
Rad21/scc1 is expressed in mitotic/meiotic cells of testis
To examine the expression of Rad21/Scc1 in mouse testicular germ cells, we used sedimentation at unit gravity to obtain cell fractions enriched for spermatogonia and meiotic prophase spermatocytes (Bellve et al, 1977). The cell purity of each fraction was higher than 80% as assessed by microscopy.
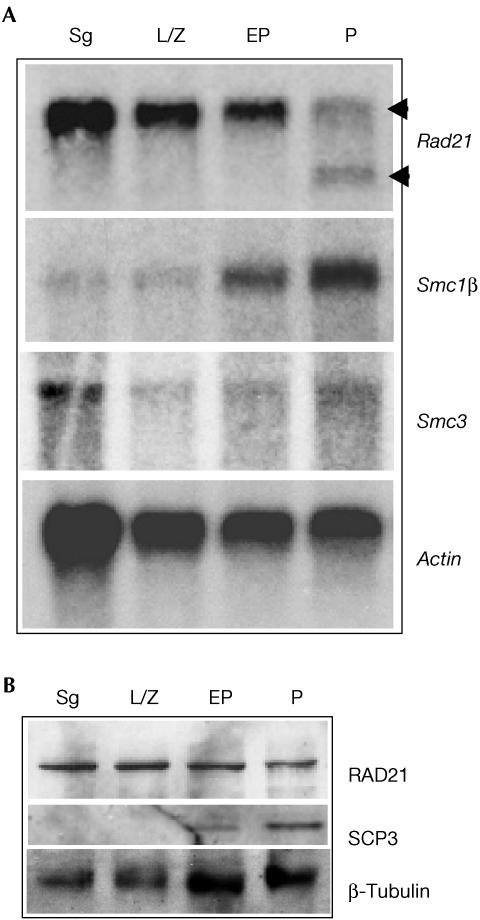
RNA blot analysis detected a 3.1-kb Rad21/Scc1 transcript in spermatogonia (mitotic cells) and spermatocytes of all three meiotic prophase stages tested (Fig 2A). Consistent with a previous study by McKay et al (1996), an additional transcript of approximately 2.2 kb was detected in mid- to late-pachytene spermatocytes. The same blot was probed to detect mRNA for SMC1β, a meiosisspecific cohesin, which showed an increase in signal intensity in the later stages of prophase (pachytene) (Fig 2A). SMC3 cohesin, which is present in both mitotic and meiotic cells, was expressed in all types of cells examined but predominantly in spermatogonia (Fig 2A). Western blots revealed the presence of RAD21/SCC1 protein in mitotically proliferating spermatogonia as well as in all three types of prophase I spermatocytes (Fig 2B and supplementary information online). The synaptonemal complex protein SCP3 was detected predominantly in pachytene spermatocytes but not in spermatogonia, as expected. Collectively, these data demonstrated the presence of RAD21/SCC1 in meiotic cells.
Figure 2.
Expression of RAD21/SCC1 in mouse testicular cells. Testicular cells were enriched for spermatogonia (Sg), leptotene/zygotene (L/Z), early pachytene (EP) and pachytene (P) spermatocytes. (A) RNA blots probed for RAD21/SCC1, SMC3 and the meiosisspecific SMC1β. Note the presence of two different RAD21/SCC1 transcripts (arrowheads) in pachytene spermatocytes. Actin serves as a loading control. (B) Western blots probed with RAD21 Ab2. Anti-SCP3 antibody was used to verify the enrichment of pachytene spermatocytes. Loadings were verified using β-tubulin.
Rad21/scc1 associates with prophase I chromosomes
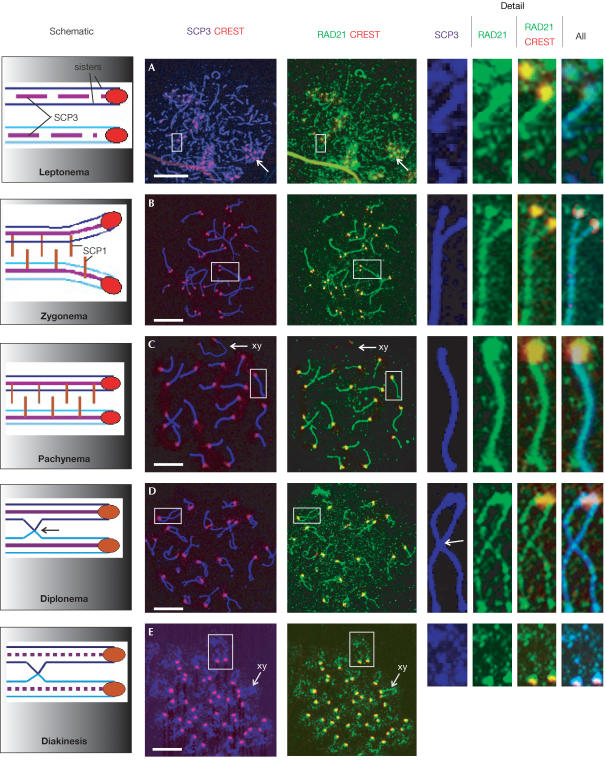
We performed a detailed examination of the localization of RAD21/SCC1 on chromosome spreads of mouse spermatocytes by immunostaining using two independent polyclonal antibodies against human RAD21/SCC1. To ensure the signal specificity, we documented the staining pattern of both antibodies separately with appropriate controls and a third antibody against human SCC1 (supplementary information online). We then costained spreads with an antibody against SCP3 protein to assist in identifying the different stages of meiotic progression. SCP3 (also known as SYCP3) is a component of the axial/lateral elements (AEs/LEs) of the synaptonemal complex (SC), a tripartite proteinaceous structure formed between homologous chromosomes during meiotic prophase I (Heyting, 1996). SCP3 has distinct chromosomal distributions at different stages of meiosis, and is commonly used for marking the progression of meiotic prophase I (Dobson et al, 1994; Schalk et al, 1998). Various controls were performed in co-immunostaining (supplementary Figs 10 and 11 online). During prophase I, SCP3 is first seen in short stretches of thin fibres at leptonema, when AEs begin to form (Fig 3A). RAD21/SCC1 was detected in rows of dots, which localized along the AEs, judging by the position of SCP3 staining (Fig 3A). Centromeric localization of RAD21/SCC1 was also observed (Fig 3A), as determined by costaining of RAD21/SCC1 with CREST-6, which primarily stains kinetochore proteins. As cells progressed to zygonema, SCP3 staining became more continuous as the AEs extended and synapsis proceeded between homologous chromosomes in late zygonema (Fig 3B). RAD21/SCC1 was detected as discrete and mainly contiguous foci along both unsynapsed and synapsed regions of chromosomes (Fig 3B). Here, RAD21/SCC1 was also clearly present on centromeres where it colocalized with the majority of the CREST signal (Fig 3B). At pachynema, when homologous chromosomes have completed synapsis, RAD21/SCC1 became contiguous on the axes of autosomes (Fig 3C). Centromeric RAD21/SCC1 staining again colocalized with the CREST staining (Fig 3C). Although SCP3 staining was clear, we noted that the XY chromosomes had a much weaker RAD21/SCC1 signal in early pachynema (Fig 3C). RAD21/SCC1 intensity appeared to decrease in mid-pachynema as chromosomes become condensed (supplementary Fig 7C online). This is consistent with our western blot analysis, which showed a reduction of RAD21 protein in mid- to late-pachytene spermatocytes (Fig 2 and supplementary Fig 5 online). An increase in RAD21/SCC1 intensity was observed on the sex body at late pachynema (data not shown). At diplonema, RAD21/SCC1 intensity decreased and it appeared as punctate foci along desynapsed chromosome arms while it remained largely on centromeres and telomeres (Fig 3D; supplementary Figs 7D, 8D and 9D online). The presence of RAD21/SCC1 along chromosome arms was previously reported (Prieto et al, 2002). By diakinesis, SCP3 remained on centromeres but became more discontinuous along the chromosome axes (Fig 3E; Dobson et al, 1994). RAD21/SCC1, colocalizing completely with SCP3, showed strong staining on centromeres, whereas a small amount remained on the arms (Fig 3E).
Figure 3.
RAD21/SCC1 associates with chromosomal arms and centromeres during meiotic prophase I. Schematic on the far left depicts the major elements of chromosome arm and centromere behaviour in temporal order in meiotic prophase I. Chromosome spreads of mouse spermatocytes were immunostained using antibodies against SCP3 (purple), CREST (staining for centromeric proteins, red) and RAD21/SCC1 (green). The merged images have the following colours for colocalization: RAD21/SCC1 and CREST (yellow); RAD21/SCC1 and SCP3 (vivid blue); and all three proteins (light blue). Representative cells in the five major stages of meiotic prophase I are shown in temporal order: (A) leptonema, with arrows indicating a typical cluster of centromeres; (B) late zygonema; (C) pachynema, with arrows indicating the XY chromosomes (note the absence of RAD21/SCC1 staining on the XY chromosomes); (D) diplonema, with an arrow indicating the site of a crossover; and (E) diakinesis (note that more SCP3 and RAD21/SCC1 are retained on the X chromosomes) (arrows). Detailed views of chromosomes (far right; not to scale) were obtained from the white boxes on the main panels, and are shown from left to right in the order of: SCP3; RAD21/SCC1 alone; RAD21/SCC1 and CREST merged; RAD21/SCC1, SCP3 and CREST merged. Scale bar, 10 μm.
Our data clearly show that RAD21/SCC1 associates with meiotic axial cores and centromeres throughout prophase I in mouse spermatocytes. Cohesin REC8 has an essential role in the formation of SC in budding yeast and Caenorhabditis elegans (Klein et al, 1999; Pasierbek et al, 2001). In mammals, cohesins including SMC3, SMC1β, STAG3, REC8 and RAD21/SCC1 appear to associate with the components of the SC (Eijpe et al, 2000; Prieto et al, 2001; Revenkova et al, 2001; Eijpe et al, 2003; Lee et al, 2003). It remains to be determined whether mammalian cohesins have a similar role in the formation of the synaptomenal complex. When expressed under the control of the Rec8 promoter, budding yeast SCC1 was unable to support SC formation in the absence of REC8, suggesting that RAD21/SCC1 is not essential for the formation of the SC (Toth et al, 2000). However, it was able to establish SCC (Toth et al, 2000). Similarly, RAD21 appeared to be capable of maintaining SCC in a fission yeast mutant lacking Rec8 (Watanabe & Nurse, 1999). Our results show that the pattern of chromosomal distribution of RAD21/SCC1 is consistent with a role for RAD21/SCC1 in SCC in meiosis.
Rad21/scc1 persists on centromeres until anaphase II
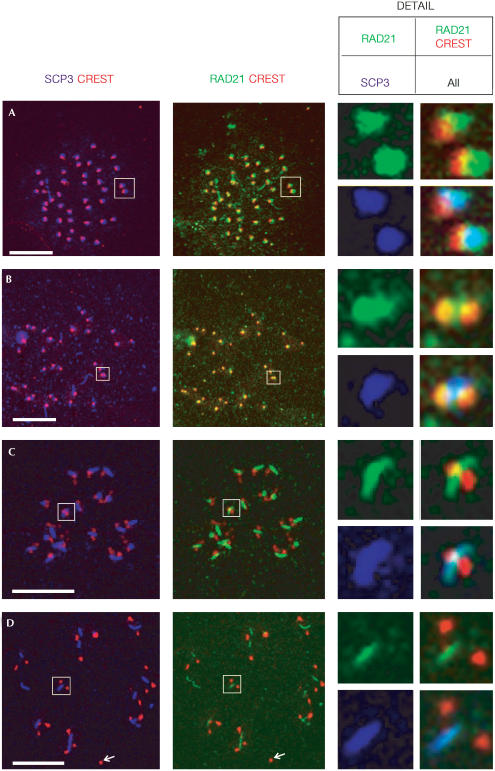
The observation that RAD21/SCC1 is present at the centromeres of MI chromosomes is of considerable interest, as the persistence of RAD21/SCC1 on centromeres until the metaphase-to-anaphase transition is essential for proper chromosome segregation in mitosis (Uhlmann et al, 1999; Waizenegger et al, 2000). Our data suggest a similar requirement for RAD21/SCC1 in meiotic chromosome segregation in mammals. We further examined RAD21/SCC1 localization on metaphase I and II chromosome spreads. We gave careful attention to the localization of RAD21/SCC1 antibody with respect to that of antisCP3 (which stains the centromeres in metaphase I and II spermatocytes; Revenkova et al, 2001; Eijpe et al, 2003), and the CREST antiserum, detecting centromeric proteins. At metaphase I, the kinetochores of sister chromatids are tightly associated and 40 dots, representing centromeres of individual chromosomes, can be visualized by CREST staining (Fig 4A; Revenkova et al, 2001). Partial colocalization was observed between SCP3 staining and the CREST signal (Fig 4A). At this stage, RAD21/SCC1 colocalized completely with SCP3 and a portion of it overlapped with the CREST staining (Fig 4A). In anaphase I, sister centromeres can be visualized as two individual dots by CREST staining (Prieto et al, 2001). RAD21 partly colocalized with CREST and was also present on the region between the two dots of CREST staining (Fig 4B). This localization is consistent with what is expected for proteins involved in SCC. At metaphase II, approximately 20 rodshaped SCP3 signals were observed between two close dots of CREST staining (Fig 4C; Dobson et al, 1994; Eijpe et al, 2003). RAD21 signal overlapped with SCP3, situating in the region between two dots of CREST signal (Fig 4C). Colocalization of RAD21/SCC1 with CREST was restricted to the region bordering sister centromeres, suggesting that RAD21/SCC1 occupied a centromeric region partly different from the proteins recognized by CREST (Fig 4C). This centromeric distribution of RAD21/SCC1 at metaphase II closely resembles the RAD21/SCC1 localization observed in mitotic metaphase cells (Waizenegger et al, 2000; Hauf et al, 2001; this study). When cells enter into anaphase II, the rodshaped SCP3 staining is still detectable but the number of rod-shaped SCP3 aggregates is reduced (Revenkova et al, 2001; Eijpe et al, 2003). The RAD21/SCC1 signal appeared to be reduced in anaphase II cells, but was still retained between two CRESTstaining sister centromeres (Fig 4D). Here, no overlap between RAD21/SCC1 and CREST was observed, indicating that the majority, if not all, of RAD21/SCC1 is released from centromeres at metaphase II (Fig 4D). The residual RAD21/SCC1 was detected at the spindle midzone during anaphase of mitosis and was implicated in the completion of cytokinesis (Hauf et al, 2001; Hoque & Ishikawa, 2001). We speculate that the retention of RAD21/SCC1 in the rodshaped structure, along with SCP3, might have a similar function.
Figure 4.
RAD21/SCC1 persists at the centromeres of meiotic chromosomes until anaphase II. Metaphase I to anaphase II chromosome spreads of spermatocytes were immunostained with antibodies against SCP3 (purple), CREST (staining for kinetochores, red) and RAD21 (green). (A) Metaphase I, (B) anaphase I, (C) metaphase II and (D) metaphase II/anaphase II; the arrow indicates the loss of SCP3 and RAD21/SCC1 staining from centromeres. Detailed views of chromosomes (boxed, not to scale) are RAD21/SCC1 alone (top left panel), RAD21/SCC1 and CREST merged (top right panel), SCP3 alone (bottom left panel) and all three proteins merged (bottom right panel). Colocalization of RAD21 with CREST is yellow in a merged image. Light blue indicates colocalization of all three proteins. Scale bar, 10 μm.
Rad21/scc1 in meiosis: functional implications
This study unambiguously demonstrates an association of RAD21/SCC1 with key chromosomal events during mammalian meiosis. Of particular interest is the association of RAD21/SCC1 with meiotic chromosomal arms during MI, and its persistence at centromeres until anaphase II. The sequential loss of RAD21/SCC1 from chromosomal arms and centromeres supports a role for mammalian RAD21/SCC1 in maintaining meiotic SCC and chromosome segregation, a function performed by REC8 in yeast (Klein et al, 1999). The question arises as to how the two proteins, RAD21/SCC1 and REC8, coordinate their respective contributions to provide SCC during meiosis. Some important clues come from studies of fission yeast REC8 (Kitajima et al, 2003; Yokobayashi et al, 2003). In fission yeast, replacement of REC8 with RAD21/SCC1 on centromeres resulted in equational segregation at MI, strongly suggesting that REC8 is essential for establishing the typical monopolar attachment of kinetochores to spindle microtubules in MI, whereas RAD21/SCC1 defines bipolar attachment (Yokobayashi et al, 2003). Interestingly, centromeric REC8 in fission yeast appeared to associate with two different subcentromeric domains, which are implicated in cohesion during MII and in establishing monopolar attachment of kinetochores to microtubule spindles in MI (Kitajima et al, 2003). The same principle could hold for mammals. Our data unambiguously show that mammalian RAD21/SCC1 localizes at centromeres during MII. In contrast, mammalian REC8 was found to localize at two centromeric sites flanking kinetochores and the SCP3-binding region in MII (Eijpe et al, 2003). Thus, the two proteins appear to localize at different centromeric domains. Furthermore, our analysis of mice lacking Rec8 revealed that centromeric cohesion is maintained in the absence of REC8 (our unpublished data). Collectively, these data suggest that in mammals, although REC8 may remain responsible for SC formation and monopolar attachment of kinetochores to microtubule spindles in MI, its role(s) in maintaining centromeric SCC and in regulating chromosome segregation in MII may have been (partly) replaced by its paralogous protein RAD21/SCC1. Presumably, RAD21/SCC1 might function in establishing bipolar attachment required for the equational segregation of sister chromatids in MII. This proposition is consistent with recent reports that mammalian RAD21/SCC1 is required for the association of kinetochores with microtubules and the binding of some centromeric proteins during mitosis (Morrison et al, 2003).
Our results show that the temporal and spatial distribution of RAD21/SCC1 is similar to that described for SMC3 and the meiosis-specific cohesin SMC1β (Eijpe et al, 2000; Revenkova et al, 2001) but differs from STAG3 (the meiotic paralogue of SCC3), SMC1α and REC8 (Prieto et al, 2001; Eijpe et al, 2003; Lee et al, 2003). Further analysis using immunoelectron microscopy will be required to obtain fine structural information on colocalization of cohesins. We speculate that at least three different cohesin assemblies may exist in mammalian meiosis. A complex containing RAD21/SCC1–SMC1β–SMC3 is presumably involved in SCC and MII kinetochore function. In addition, a REC8–SMC1β–SMC3 complex may function in MIspecific events, including SC formation and monopolar spindle attachment, and in MII centromeric cohesion. Furthermore, SMC1α–SMC3, possibly RAD21/SCC1 and other unidentified cohesins may be responsible for maintaining chromatin loop cohesion.
Speculation
The persistence of centromeric RAD21/SCC1 during MII and its loss at anaphase II closely resembles its behaviour during mitosis, where loss of centromeric RAD21/SCC1 at the metaphase-to-anaphase transition facilitates segregation of sister chromatids (Uhlmann et al, 1999). Although not identical, MII has long been thought to be comparable to mitosis, as both divisions involve the equational segregation of sister chromatids (Nasmyth, 2001). Our data provide the first molecular evidence that in mammals the cellular machinery responsible for chromosome segregation during these two types of equational cell division could be fundamentally similar.
The removal of RAD21/SCC1 from chromosome arms at prophase and from centromeres at the onset of anaphase depends on a Polo-like kinase (PLK) and separase, respectively, in vertebrate mitosis (Waizenegger et al, 2000). The specific expression of murine PLK1 at diplonema and diakinesis in mouse spermatocytes (Matsubara et al, 1995) corresponds with the timing of RAD21/SCC1 disappearance from chromosomal arms as observed in this study, suggesting that similar regulation of segregation might exist in both mitotic and meiotic cells.
Methods
Both anti-RAD21 antibodies were used at 20 μg/ml for immunostaining. Fig 1 was obtained using RAD21 Ab2, and Figs 3 and 4 were generated using RAD21 Ab1. Controls were performed in parallel by omitting primary antibodies and using pre-immune sera. Primary antibodies used were rabbit antisCP3, rabbit anti-SCC1, anti-γH2AX and human CREST-6 sera. The secondary antibodies (Molecular Probes) used were Alexa 488 anti-sheep IgG, Alexa 568 anti-rabbit IgG and Alexa 647 anti-human IgG. Data were collected on a confocal microscope (Bio-Rad MRC 1000). Images were manipulated using Confocal Assistant™ 4.02 and Adobe Photoshop software. See supplementary methods online for more information.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v5/n4/extref/7400121s1.pdf).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8
Supplementary Figure 9
Supplementary Figure 10
Supplementary Figure 11
Supplementary Methods
Acknowledgments
We thank Dr R. Jessberger for the gift of anti-SMC3, Dr C. Heyting for anti-SCP3, Dr A. Choo for CREST-6, Dr J.M. Peters for anti-SCC1, Dr W.M. Bonner for anti-γH2AX antibodies and S. Ellis for help with microscopy. This work was supported by grants no. 9936643 and no. 251686 to M.J.M. from the Australian National Health and Medical Research Council, and no. HD33816 to M.A.H. from the National Institutes of Health.
References
- Bellve AR et al. (1977) Spermatogenic cells of the prepuberal mouse. J Cell Biol 74: 68–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson MJ, Pearlman RE, Karaiskakis A, Spyropoulos B, Moens PB (1994) Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J Cell Sci 107: 2749–2760 [DOI] [PubMed] [Google Scholar]
- Eijpe M, Heyting C, Gross B, Jessberger R (2000) Association of mammalian SMC1 and SMC3 proteins with meiotic chromosomes and synaptonemal complexes. J Cell Sci 113: 673–682 [DOI] [PubMed] [Google Scholar]
- Eijpe M, Offenberg H, Jessberger R, Revenkova E, Heyting C (2003) Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1β and SMC3. J Cell Biol 160: 657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S, Waizenegger IC, Peters JM (2001) Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science 293: 1320–1323 [DOI] [PubMed] [Google Scholar]
- Heyting C (1996) Synaptonemal complexes: structure and function. Curr Opin Cell Biol 8: 389–396 [DOI] [PubMed] [Google Scholar]
- Hoque MT, Ishikawa F (2001) Human chromatid cohesin component hRad21 is phosphorylated in M phase and associated with metaphase centromeres. J Biol Chem 276: 5059–5067 [DOI] [PubMed] [Google Scholar]
- Jessberger R (2002) The many functions of SMC proteins in chromosome dynamics. Nat Rev Mol Cell Biol 3: 767–778 [DOI] [PubMed] [Google Scholar]
- Kitajima TS, Yokobayashi S, Yamamoto M, Watanabe Y (2003) Distinct cohesin complexes organize meiotic chromosome domains. Science 300: 1152–1155 [DOI] [PubMed] [Google Scholar]
- Klein F et al. (1999) A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell 98: 91–103 [DOI] [PubMed] [Google Scholar]
- Lee J, Iwai T, Yokota T, Yamashita M (2003) Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J Cell Sci 116: 2781–2790 [DOI] [PubMed] [Google Scholar]
- Matsubara N, Yanagisawa M, Nishimune Y, Obinata M, Matsui Y (1995) Murine polo like kinase 1 gene is expressed in meiotic testicular germ cells and oocytes. Mol Reprod Dev 41: 407–415 [DOI] [PubMed] [Google Scholar]
- McKay MJ et al. (1996) Sequence conservation of the rad21 Schizosaccharomyces pombe DNA doublestrand break repair gene in human and mouse. Genomics 36: 305–315 [DOI] [PubMed] [Google Scholar]
- Morrison C, Vagnarelli P, Sonoda E, Takeda S, Earnshaw WC (2003) Sister chromatid cohesion and genome stability in vertebrate cells. Biochem Soc Trans 31: 263–265 [DOI] [PubMed] [Google Scholar]
- Nasmyth K (2001) Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu Rev Genet 35: 673–745 [DOI] [PubMed] [Google Scholar]
- Pasierbek P, Jantsch M, Schleiffer A, Schweizer D, Loid J (2001) A Caenorhabditis elegans cohesin protein with functions in meiotic chromosome pairing and disjunction. Genes Dev 15: 1349–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto I et al. (2001) Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat Cell Biol 3: 761–766 [DOI] [PubMed] [Google Scholar]
- Prieto I et al. (2002) STAG2 and Rad21 mammalian mitotic cohesins are implicated in meiosis. EMBO Rep 3: 543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenkova E, Eijpe M, Heyting C, Gross B, Jessberger R (2001) Novel meiosisspecific isoform of mammalian SMC1. Mol Cell Biol 21: 6984–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk JA et al. (1998) Localization of SCP2 and SCP3 protein molecules within synaptonemal complexes of the rat. Chromosoma 107: 540–548 [DOI] [PubMed] [Google Scholar]
- Toth A et al. (2000) Functional genomics identifies monopolin: a kinetochore protein required for segregation of homologs during meiosis I. Cell 103: 1155–1168 [DOI] [PubMed] [Google Scholar]
- Uhlmann F, Lottspeich F, Nasmyth K (1999) Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400: 37–42 [DOI] [PubMed] [Google Scholar]
- Waizenegger IC, Hauf S, Meinke A, Peters JM (2000) Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103: 399–410 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nurse P (1999) Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature 400: 461–464 [DOI] [PubMed] [Google Scholar]
- Yokobayashi S, Yamamoto M, Watanabe Y (2003) Cohesins determine the attachment manner of kinetochores to spindle microtubules at meiosis I in fission yeast. Mol Cell Biol 23: 3965–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8
Supplementary Figure 9
Supplementary Figure 10
Supplementary Figure 11
Supplementary Methods