Abstract
Recently, we have shown that (S)-N′-Nitrosonornicotine [(S)-NNN], the major form of NNN in tobacco products, is a potent oral cavity and esophageal carcinogen in rats. To determine the early molecular alterations induced by (S)-NNN in the oral and esophageal mucosa, we administered the carcinogen to rats in the drinking water for 10 weeks and global gene expression alterations were analyzed by RNA sequencing. At a false discovery rate p-value < 0.05 and fold-change ≥ 2, we found alterations in the level of 39 genes in the oral cavity and 69 genes in the esophagus. Validation of RNA sequencing results by qRT-PCR assays revealed a high cross-platform concordance. The most significant impact of exposure to (S)-NNN was alteration of genes involved in immune regulation (Aire, Ctla4 and CD80), inflammation (Ephx2 and Inpp5d) and cancer (Cdkn2a, Dhh, Fetub B, Inpp5d, Ly6E, Nr1d1 and Wnt6). Consistent with the findings in rat tissues, most of the genes were deregulated, albeit to different degrees, in immortalized oral keratinocytes treated with (S)-NNN and in non-treated premalignant oral cells and malignant oral and head and neck squamous cells. Furthermore, interrogation of TCGA data sets showed that genes deregulated by (S)-NNN in rat tissues (Fetub, Ly6e, Nr1d1, Cacna1c, Cd80 and Dgkg) are also altered in esophageal and head and neck tumors. Overall, our findings provide novel insights into early molecular changes induced by (S)-NNN and therefore could contribute to the development of biomarkers for the early detection and prevention of (S)-NNN-associated oral and esophageal cancers.
Keywords: N′-nitrosonornicotine, RNA sequencing, gene expression, soluble epoxide hydrolase
Introduction
Accumulating evidence indicates that N′-nitrosonornicotine (NNN), a tobacco-specific carcinogen present in substantial amounts in cigarette smoke, smokeless tobacco products and unburned tobacco, plays an important role in the induction of cancers of the esophagus and oral cavity. NNN causes esophageal, oral cavity, and nasal cavity tumors in rats, nasal cavity tumors in mink, and respiratory tract tumors in mice and hamsters [1, 2]. Oral swabbing with a mixture of NNN and the related tobacco nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induced tumors in the oral cavity of rats [3]. In 2007, the International Agency for Research on Cancer (IARC) classified NNN and NNK as human carcinogens [4].
NNN, with a chiral center at its 2′-position, exists as two enantiomers: (S)-NNN and (R)-NNN. (S)-NNN is the predominant enantiomer in currently marketed smokeless tobacco products, comprising 57–66% of total NNN in these products [5–7]. Our earlier studies demonstrated that (S)-NNN was metabolized in rats by 2′-hydroxylation, a known pathway leading to pyridyloxobutyl (POB)-DNA adduct formation, to a significantly greater extent than (R)-NNN in the esophagus [8]. Moreover, DNA adduct formation from (S)-NNN significantly exceeded that from (R)-NNN in the esophagus, oral cavity, and liver of F-344 rats treated with the compounds in the drinking water for 20 weeks [9, 10]. These studies strongly suggested that (S)-NNN would be more carcinogenic than (R)-NNN to the rat esophagus and that the oral cavity might also be a target tissue for (S)-NNN. Indeed, in our recent studies with male F-344 rats treated chronically with 14 ppm (S)-NNN in the drinking water, we showed that (S)-NNN is a strong oral cavity carcinogen, whereas the opposite enantiomer (R)-NNN was inactive, but synergistically enhanced the carcinogenicity of (S)-NNN [2].
Although (S)-NNN is a potent esophageal and oral cavity carcinogen in rats, the molecular mechanisms responsible for its carcinogenicity and its higher potency as compared to (R)-NNN are not known. Therefore, in the study presented here, we sought to determine, using RNA sequencing, the gene expression profile of oral and esophagus tissues from rats chronically treated with (S)-NNN in the drinking water for 10 weeks. Given that (S)-NNN is an important risk factor for both oral and esophageal cancers, the results of these studies might yield insights into molecular alterations induced during the early stage of oral and esophageal carcinogenesis, which subsequently could be used as biomarkers for early cancer detection and targets for chemoprevention.
Materials and Methods
Synthesis and characterization of NNN enantiomers
Nornicotine enantiomers were prepared as described [11], nitrosated and purified by column chromatography on silica gel, providing the pure NNN enantiomers [5]. Specific rotations of the enantiomers were described previously [5]. Purity, as assessed by HPLC with UV detection, was 96.5% for (S)-NNN and >99% for (R)-NNN.
Cell lines and culture conditions
Normal oral keratinocytes (NHOK), immortalized by Bmi-1 and HPV-16 E6 [12], were obtained from Dr. Cherie-Ann O. Nathan, at LSU Health Shreveport, Shreveport, LA, which were originally from Dr. Karl Munger at Harvard Medical School/Brigham and Women’s Hospital, Boston, MA, USA. Oral keratinocyte OKF6-TERT1 cells, derived from a biopsy of normal floor of mouth, were obtained from Dr. James G. Rheinwald (Brigham and Women’s Hospital, Harvard Institutes of Medicine Boston, MA) in February 2015. These cells are immortalized, but not transformed and stably express hTERT, the catalytic subunit of telomerase [13]. Both NHOK and OKF6 were cultured in keratinocyte-SFM (KSFM) media supplemented with 25μg/ml bovine pituitary extract and 5ng/ml EGF (supplied with the medium).
Human oral leukoplakia (Msk-Leuk1) cells, obtained from Dr. Frank G. Ondrey (University of Minnesota, MN), were derived from a dysplastic leukoplakia lesion located adjacent to a squamous cell carcinoma (SCC) of the tongue. These cells do not produce tumors when implanted in nude mice, although they exhibit anchorage independent growth [14]. The authenticity of these cells was confirmed in 2011 by short tandem repeat (STR) genotyping at the Genetic Resources Core Facility at Johns Hopkins University. Msk-Leuk1 cells were cultured in KGM with a bullet kit (keratinocyte growth medium; Lonza).
Human HNSCC cell lines TR146, SCC-58 and UM-SCC-17B were all kindly provided by Dr. Mark Herzberg (University of Minnesota). TR146 and SCC-58 cells were established from a cervical lymph node metastasis of a well-differentiated buccal carcinoma and primary tumors of the oral cavity, respectively. UM-SCC-17B cells were established from original neck tumors. All cell lines were tested for mycoplasma and HPV infection and found to be negative. These cells were grown in Dulbecco’s Modified Eagle Medium/Ham’s F-12 (DMEM/F-12) media supplemented with 10% heat inactivated fetal calf serum and maintained in 5% CO2 at 37°C.
Exposure of OKF-6 cells to (S)-NNN or (R)-NNN
Exponentially growing OKF-6 cells were plated in a 60 mm dish at a density of 1.5 x 105 cells per dish, one day prior to treatment with the carcinogens, (S)-NNN or (R)-NNN were dissolved in DMSO and diluted in the culture media. Subsequently, the cells were treated with either of the carcinogens at a concentration of 1, 10, 100 or 1000 μM for three days. Carcinogen treatment was repeated for four cycles with replacement of the old media with fresh media containing the carcinogens every time. At the end of the treatment, the cells were harvested, RNA isolated and used for downstream assays.
Treatment of male F-344 rats with (S)-NNN
This study was approved by the University of Minnesota Institutional Animal Care and Use Committee. Male F-344 rats, age 6 weeks, were obtained from Charles River Laboratories (Kingston, NY), housed 2 per cage with Harlan-irradiated Corncob bedding (Harlan, Indianapolis, IN) and allowed to acclimate to the Research Animal Resources Facility, University of Minnesota, for 1 week. The rats were maintained under standard conditions (20–24 °C, 29–32% relative humidity and 14/10 light/dark cycle) and maintained on NIH-07 diet and tap water. The study was performed essentially as described before [2]. Briefly, after one week of acclimatization, 12 male F-344 rats were randomly assigned, 6 rats/group, into control and (S)-NNN groups. Rats in the (S)-NNN group received the carcinogen in the drinking water at a concentration of 14 ppm for 10 weeks, whereas rats in the control group were given plain tap water. In our previous studies in which we quantified DNA adducts resulting from (S)-NNN) treatment of male F-344 rats for 10, 30, 50, and 70 weeks, we observed a significantly increased DNA adduct level as early as week 10. Therefore, this time point was used for gene expression studies. Moreover, we were interested in early changes so as to target these alterations with chemopreventive agents. At the end of the study, rats were euthanized with an overdose of carbon dioxide and esophagi and the heads were harvested. Subsequently, esophageal mucosa and oral mucosa were isolated and used for the preparation of RNA.
RNA extraction and quality control
Cells or rat esophageal and oral mucosa tissues were homogenized in TRIzol® using a Polytron® System PT 1200C (Kinematica AG, Switzerland), and total RNA extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. The concentration (A260) and purity (A260/A280 and A260/A230) of RNA were determined using NanoDrop 1000 spectrophotometry. RNA integrity was confirmed using an Agilent Bioanalyzer and/or Caliper GX. Only RNA samples with RIN higher than 8 were selected for RNA sequencing.
Library preparation and RNA sequencing
Library preparation and RNA sequencing were performed as previously described [15] using TruSeq RNA v2 library preparation reagents (with average insert size of ~ 200 bp) and sequenced at twenty million read depth (± 10%) on an Illumina HiSeq 2000 50bp Paired-End sequencer for 50 cycles. Average quality scores (Q-score) for all libraries above 30 for both reads 1 and 2 were selected for further analyses.
RNA sequencing data and statistical analysis
Paired-End RNA sequencing data were analyzed using CLC Bio Genomics Workbench 7 (CLC Bio, Qiagen, Boston, MA) as previously described [15] for quality control, mapping, and expression analyses. Sequencing data that passed quality control analysis were mapped to rat reference genome version 5 (Rnor_5) using Ensembl annotation version Rnor_5.0.77 with default settings. Maximum number of hits for each read was set to 10 and total exon counts at the gene level were exported for differential expression analysis using DESeq [16] in R data analysis software (www.r-project.org). Statistical significance was calculated using both p-value and false discovery rate (FDR) < 0.05 and fold-change ≥ 2.0-fold as cutoffs. Fold-change was determined as the ratio of means of NNN to control treatment. Fold down-regulation with ratio of means less than 1.0 is presented as −1/(ratio of means). Pathway analysis and gene ontology (Ingenuity Pathways Analysis; IPA, Ingenuity Systems, Inc., Redwood City, CA) were performed to identify putative functions associated with (S)-NNN deregulated genes.
TCGA RNA sequencing data and statistical analysis
To determine whether genes significantly regulated by (S)-NNN in rat esophageal and oral cavity tissues were also regulated in human esophageal carcinoma (ESCA) and head and neck squamous cell carcinoma (HNSCC), respectively, RNA-Seq data from The Cancer Genome Atlas (TCGA) Network (http://cancergenome.nih.gov) were further interrogated. Level 3 RNA sequencing version 2 (RNA-Seq V2) datasets from 185 ESCA cases that included 11 normal adjacent and 185 ESCA tumor tissues and from 528 HNSCC cases (43 normal adjacent and 521 tumor tissues) were analyzed. Briefly, gene expression raw counts representing total transcripts mapped to the hg19 human reference genome using MapSplice alignment and quantitated by RNA-Seq by expectation-maximization (RSEM) [17,18] were imported and processed using R data analysis software (www.r-project.org). Normalization (Trimmed Mean of M-values, or TMM method) and differential expression analysis were performed using edgeR Bioconductor package [19] since the RSEM count data were incompatible with DESeq package. Statistical significance was calculated using false discovery rate (FDR) < 0.05 and fold-change ≥ 2.0 as cutoffs. Fold-change was determined as the ratio of means of tumor to normal samples. Fold down-regulation with ratio of means less than 1.0 is presented as described above. Genes significantly regulated by (S)-NNN in rat esophageal and oral cavity tissues that were also significantly regulated in human ESCA and HNSCC, respectively, in the same direction in comparison to corresponding normal tissues were reported.
Copy number alterations and mutational analysis from TCGA
Genes deregulated by (S)-NNN were interrogated against the human esophageal carcinoma (ESCA) and head and neck squamous cell carcinoma (HNSCC) provisional datasets publically available from The Cancer Genome Atlas (TCGA) [17] using the web-based cBioPortal [20] [data mining interface (www.cbioportal.org)]. Briefly, gene sets of interest were submitted through cBioPortal and putative copy number alternations (CNA) data from Genomic Identification of Significant Targets in Cancer (GISTIC) [20] for each gene were retrieved and presented in a graphical OncoPrints, showing putative homozygous (deep) genetic deletions and genetic amplification (gain in two or more copy numbers). CNA data were interrogated in 186 samples from 185 ESCA cases and in 530 samples from 528 HNSCC cases. In addition, somatic mutation data also were interrogated in HNSCC samples. ESCA somatic mutation data were not available through cBioPortal at the time of analysis.
Quantitative RT-PCR analysis
We validated, by qRT-PCR, the expression level of 56 genes in both esophageal and oral mucosa RNA samples used for RNA sequencing studies. Additionally, we analyzed the expression of 14 of these genes in (S)-NNN- or (R)-NNN-treated immortalized OKF6 keratinocytes and 20 genes in untreated OKF6, Msk-Leuk1, TR146, UM-SCC-17B, and SCC-58 cell lines. qRT-PCR assays were essentially performed as described by us previously [15] using gene-specific primers (Supplementary Tables 1A and 1B).
Western blot analysis of mouse lung tissues and cell lines
For the analysis of rat esophageal and oral mucosa, tissues from three rats were homogenized in a 1× RIPA buffer containing protease and phosphatase inhibitors. For the assay with cell lines, OKF6, Msk-Leuk1, TR146, SCC-58 and UM-SCC-17B, ~1×106 cells were suspended in the RIPA buffer containing protease and phosphatase inhibitors on ice for 1hr. Subsequently, tissue or cell lysates were centrifuged (14000 g for 25 min at 4°C), the supernatants collected, aliquoted and stored at −80°C. Western immunoblotting assays were performed as described previously [22]. Briefly, 20 μg of protein from cell lysates or 50 μg protein from tissue lysates were loaded on to the gel. Also, biotinylated protein ladder (Cell Signaling Technology) was included into the first-left lane of each gel and used as the molecular weight marker to detect the correct target band. Membranes were stripped and re-probed with anti-β-actin (1:1000) to check for differences in the amount of protein loaded in each lane. Immunoblotting assays were performed three times. For quantitative determination of protein levels, densitometry measurements of protein bands were performed using digitalized scientific software program UN-SCAN-IT software (Silk Scientific, Orem, UT).
Statistical analysis
Data obtained from in vitro studies are presented as mean ± standard deviation (s.d), whereas the in vivo data are shown as mean ± standard error of the mean (SEM). Difference between the “Treatment” and “Control” categories for each of the concerned genes were analyzed using a 2-sample t-test under a two-sided alternative at 5% level of significance. All analyses were conducted using the R data analysis software (www.r-project.org).
Results
Gene expression profile analysis by RNA-Seq
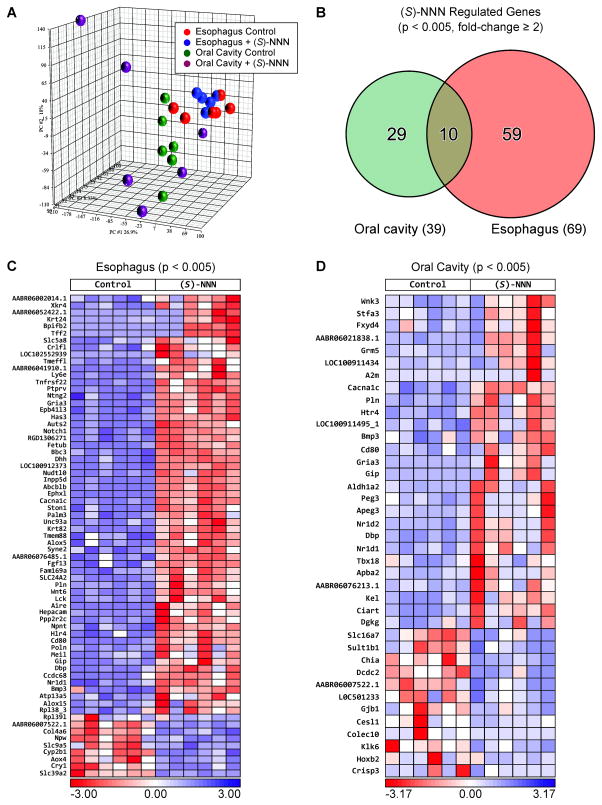
To determine the potential molecular mechanisms of (S)-NNN-induced tumor formation and identify early onset genes associated with oral cavity and esophageal carcinogenesis, we performed, genome-wide gene expression profiling of esophageal and oral mucosa from rats treated with (S)-NNN using RNA-Seq analysis. A total of 22.9 ± 2.1 million reads were compared between (S)-NNN treated and control groups at a sequencing quality score of 37 ± 0.1. A quality score of greater than or equal to 30 implies a probability of an incorrect base call of less than or equal to 1 per 1000 bp [23]. We found distinctive gene expression patterns associated with exposure to (S)-NNN in both esophageal and oral cavity tissues. While the results from esophageal mucosa were consistent, oral mucosa samples showed higher variation in gene expression patterns (Figure 1A). At a p-value of < 0.005 and fold-change ≥ 2, we found alterations in the level of 69 genes in the esophagus and 39 genes in the oral cavity (Figure 1B). Among the genes deregulated in the esophagus, 60 were upregulated and 9 were downregulated (Figure 1C), whereas in the oral mucosa, 27 genes were upregulated and 12 genes were downregulated (Figure 1D). Among the deregulated genes, 10 genes were altered in both esophageal and oral mucosa samples. The broader list of genes upregulated and downregulated by (S)-NNN in the esophageal mucosa of rats at a less stringent p-value < 0.01 is included in Supplementary Tables 2 and 3, respectively. Similar list of genes deregulated in the oral cavity at p-value < 0.01 is shown in Supplementary Table 5.
Figure 1.
Genes deregulated by (S)-NNN in rat esophagus and oral cavity. (A) Unsupervised principal component analysis (PCA) of samples based on gene expression correlation matrix. Genes with altered expression were identified based on fold-change of ≥ 2 (up- and downregulated) and p-value < 0.005 using DESeq. (B) Venn diagram showing the number of genes deregulated by (S)-NNN in the esophagus, oral cavity, and in both the esophagus and oral cavity. (C) Expression heat map of genes overexpressed (upper right panel, red shades) or downregulated genes (lower right panel, blue shades) in the esophageal mucosa of (S)-NNN-treated rats. (D) Expression heat map of genes overexpressed (upper right panel, red shades) or downregulated genes (lower right panel, blue shades) in the oral mucosa of (S)-NNN-treated rats.
Signaling pathways and molecular functions altered in esophageal and oral mucosa tissues of (S)-NNN-treated rats
Genes altered by (S)-NNN in esophageal tissue were found to be involved in cytotoxic T lymphocyte associated molecule 4 (CTLA4) signaling, cancer, cellular movement, drug metabolism, energy production, small molecular biochemistry, and cell death/survival pathways (Supplementary Table 4). In the oral cavity, (S)-NNN modulated genes were involved in nicotine metabolism, glutamate receptor signaling, cell-to-cell signaling and interaction, molecular transport, and signaling pathways associated with cell cycle, cell death, cell survival, and cell morphology (Supplementary Table 6). Cancer-focused pathway analysis showed significant upregulation of many known cancer-related genes in both the esophagus and oral cavity, but only five known cancer genes (Colec10, Gjb1, Klk6, Slc16a7, and Hoxb2) were downregulated in the oral cavity and none in the esophagus (Supplementary Tables 7 and 8).
Interrogation of genes deregulated by (S)-NNN in publicly available human esophageal carcinoma (ESCA) and head and neck squamous cell carcinoma (HNSCC) TCGA data sets
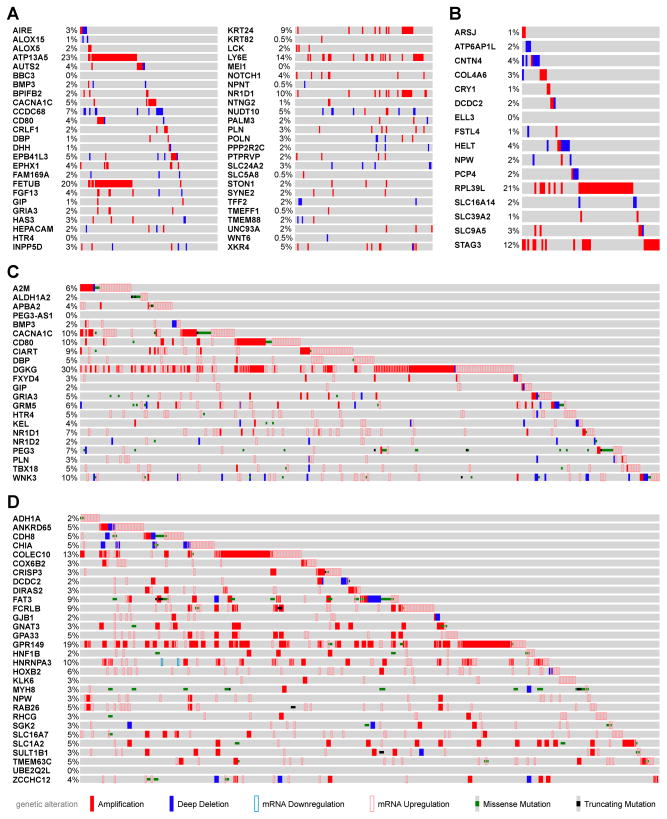
To determine if genes altered by (S)-NNN in rat oral and esophageal mucosa show the same changes in human tumors, we interrogated genes deregulated by (S)-NNN in human esophageal carcinoma (ESCA) and head and neck squamous cell carcinoma (HNSCC) datasets available from TCGA using cBioPortal (http://cbioportal.org). We found that among genes upregulated by (S)-NNN in rat esophagus with a p-value < 0.005, four genes (ATP13A5, FETUB, LY6E, and NR1D1) showed amplification of copy numbers in at least 10 – 23% of all ESCA cases analyzed (Figure 2A). At a p-value between 0.005 and < 0.05, five additional genes (ADIPOQ, B3GNT5, EMB, GPR171, and RTP4) showed increased copy numbers in 10 – 21% of esophageal carcinoma cases (Supplementary Figure 1). In addition to copy number amplifications, ATP13A5, LY6E, NR1D1, B3GNT5, EMB, and RTP4 all showed mRNA upregulation in ESCA in comparison to the adjacent normal tissues (Table 1a). Among genes that were downregulated by (S)-NNN in rat esophagus, only four genes (ATP6AP1L, CNTN4, COL4A6, and PCP4) were found to be deleted in about 2 – 4% of cases (Figure 2B) and their mRNA levels downregulated in human esophageal carcinoma (Table 1a). However, RPL39L and STAG3 genes that were downregulated by (S)-NNN were amplified in 12% and 21%, respectively, of human esophageal carcinoma cases. Similar correlations were found between genes regulated by (S)-NNN in the oral cavity of rats and human HNSCC TCGA data as summarized in Table 1b. Specifically, CACNA1C, CD80, CIART, DGKG, and WNK3 showed amplification and/or mRNA upregulation in 9–30% of the cases (Figure 2C). At 0.005 < p < 0.05, additional genes (GDF6, GLIS3, KCNB2, LRRC17, and LSAMP) exhibited amplification and/or mRNA upregulation in 8–11% of the cases (Supplementary Figure 2). Genes downregulated by (S)-NNN in the rat oral cavity rarely showed deletion in copy number (Figure 2D) but mRNA levels were reduced concordantly in human HNSCC. Interestingly, among genes downregulated by (S)-NNN in rat oral cavity, COLEC10, FCRLB, GPR149, and HNRNPA3 were amplified in 9 – 19% of HNSCC cases. Supplementary Table 9 provides a summary of genes deregulated by (S)-NNN in the rat esophagus and oral cavity and genetically altered in at least 10% of corresponding human esophageal and oral cancer tissues.
Figure 2.
cBioPortal analysis of genes deregulated by (S)-NNN in rat esophageal mucosa and oral mucosa showed similar alterations in human esophageal carcinoma and head and neck squamous cell carcinoma TCGA data sets. (A) OncoPrint of genes upregulated by (S)-NNN (p < 0.05) in rat esophagus showing copy number alteration (CNA) and/or deep deletion in human esophagus carcinoma (ESCA). (B) OncoPrint of genes downregulated by (S)-NNN (p < 0.05) in rat esophagus and showed CNA and/or deep deletion in human ESCA. (C) OncoPrint of genes upregulated by (S)-NNN (p < 0.05) in rat oral cavity and showed CNA, deep deletion, missense mutation, mRNA upregulation/downregulation, and/or truncating mutation in human HNSCC. (D) OncoPrint of genes downregulated by (S)-NNN (p < 0.05) in rat oral cavity and showed CNA, deep deletion, mRNA downregulation/upregulation, missense mutation, and/or truncating mutation in human HNSCC.
Table 1.
Genes deregulated by (S)-NNN in rat esophagus tissues are also altered in human esophageal carcinoma (ESCA) using TCGAa.
| Rat Esophagus RNA-seq | Human TCGA ESCA RNA-Seq | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Rat gene ID | Human Symbol | Fold Change | p-value | Fold Change | p-value | FDR |
| Aire | AIRE | 787.5 | 4.54E-06 | 12.2 | 8.77E-08 | 3.38E-07 |
| Mlc1 | MLC1 | 456.3 | 0.0292 | 2.8 | 7.07E-03 | 9.18E-03 |
| Mmp10 | MMP10 | 218 | 0.0157 | 27.3 | 2.24E-12 | 1.57E-11 |
| Gzmb | GZMB | 213.9 | 0.00623 | 3.9 | 3.87E-04 | 7.13E-04 |
| Klk1c8 | KLK1 | 127.4 | 0.0416 | 3.4 | 0.001214 | 0.001964 |
| Krt82 | KRT82 | 56 | 7.88E-08 | 4.8 | 3.10E-03 | 0.004492 |
| Gip | GIP | 21.7 | 0.0041 | 4.5 | 2.29E-02 | 2.54E-02 |
| Cd80 | CD80 | 16.7 | 1.68E-10 | 7.7 | 1.33E-06 | 4.19E-06 |
| Ano3 | ANO3 | 9.4 | 0.0196 | 9.0 | 3.14E-07 | 1.11E-06 |
| Nkg7 | NKG7 | 9 | 0.0157 | 2.8 | 0.005882 | 0.007847 |
| Ptchd3 | PTCHD3 | 8.2 | 0.0201 | 5.5 | 8.30E-05 | 1.77E-04 |
| Sh2d2a | SH2D2A | 6.7 | 0.0412 | 5.1 | 4.06E-05 | 9.23E-05 |
| Dlx2 | DLX2 | 6.6 | 0.0141 | 2.9 | 0.004484 | 0.006211 |
| Il27ra | IL27RA | 6.6 | 0.0308 | 2.8 | 0.005321 | 0.007195 |
| Gzma | GZMA | 6.4 | 0.0469 | 4.1 | 0.000305 | 0.000574 |
| Mx2 | MX1 | 6.4 | 0.00706 | 2.5 | 0.011965 | 0.014323 |
| Oas1a | OAS1 | 6.3 | 0.0254 | 2.5 | 0.011634 | 0.01397 |
| Skap1 | SKAP1 | 5.8 | 0.0213 | 3.4 | 1.21E-03 | 1.96E-03 |
| Oas2 | OAS2 | 4.1 | 0.0143 | 4.2 | 0.000225 | 0.000437 |
| B3gnt5 | B3GNT5 | 3.9 | 0.0314 | 2.8 | 0.005829 | 0.007787 |
| Alox15 | ALOX15 | 3.8 | 0.0044 | 33.0 | 6.39E-13 | 4.75E-12 |
| Ifit3 | IFIT3 | 3.4 | 0.0165 | 5.0 | 5.02E-05 | 1.13E-04 |
| Lck | LCK | 3.3 | 0.000497 | 3.6 | 0.000843 | 0.001424 |
| Atp13a5 | ATP13A5 | 3.2 | 0.00245 | 2.9 | 0.004273 | 0.005961 |
| Cxcl10 | CXCL10 | 3.2 | 0.0193 | 19.2 | 8.46E-11 | 4.91E-10 |
| Rsad2 | RSAD2 | 3.2 | 0.007 | 4.3 | 0.000181 | 0.00036 |
| Cxcl14 | CXCL14 | 3.1 | 0.037 | 2.7 | 0.00682 | 0.00891 |
| Spta1 | SPTA1 | 3 | 0.0074 | 2.9 | 0.013992 | 0.016495 |
| Irf7 | IRF7 | 2.9 | 0.0276 | 2.6 | 0.008675 | 0.010905 |
| Emb | EMB | 2.8 | 0.0381 | 2.2 | 0.033041 | 0.034917 |
| Bbc3 | BBC3 | 2.7 | 0.000499 | 2.7 | 8.29E-03 | 0.010534 |
| Wdr72 | WDR72 | 2.7 | 0.0293 | 10.3 | 4.78E-08 | 1.92E-07 |
| Cd7 | CD7 | 2.5 | 0.0369 | 2.7 | 7.03E-03 | 9.14E-03 |
| Nr1d1 | NR1D1 | 2.5 | 1.90E-05 | 2.0 | 4.97E-02 | 4.98E-02 |
| Usp18 | USP18 | 2.5 | 0.0395 | 3.7 | 0.00067 | 0.001164 |
| Tnfrsf18 | TNFRSF18 | 2.3 | 0.00825 | 11.6 | 1.82E-08 | 7.74E-08 |
| Ly6e | LY6E | 2.1 | 0.000698 | 5.0 | 4.99E-05 | 0.000112 |
| Rtp4 | RTP4 | 2.1 | 0.0384 | 3.2 | 0.002239 | 0.003386 |
| Col4a6 | COL4A6 | −2 | 0.00192 | −4.7 | 1.97E-10 | 1.09E-09 |
| Pcp4 | PCP4 | −2.7 | 0.0453 | −6.3 | 2.92E-15 | 2.80E-14 |
| Cntn4 | CNTN4 | −2.8 | 0.0327 | −3.7 | 2.36E-07 | 8.56E-07 |
| Atp6ap1l | ATP6AP1L | −118 | 0.0335 | −2.4 | 1.30E-03 | 2.09E-03 |
Overall, it appears that some of the genes upregulated by (S)-NNN in the rat esophageal and oral cavity model may contribute to the early onset of malignant transformation and drivers of tumorigenesis for human esophageal carcinoma and HNSCC.
qRT-PCR and immunoblotting assays corroborated RNA-Seq results
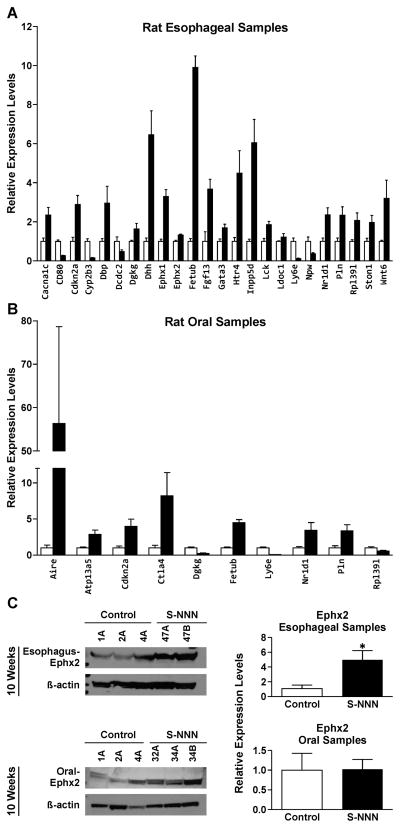
To validate the results of RNA sequencing, 56 genes were selected, on the basis of their role in carcinogenesis and magnitude of change in RNA-Seq. The genes were then analyzed by qRT-PCR in both oral and esophageal RNA samples. A high cross platform concordance was observed between RNA-Seq and qRT-PCR from our samples. However, only 24 genes were significantly altered in rat esophagus (Figure 3A), whereas only 10 genes showed significant deregulation in rat oral mucosa (Figure 3B).
Figure 3.
Validation of selected genes from RNA sequencing studies by qRT-PCR and Western immunoblotting. The expression of 56 genes was analyzed in the esophageal mucosa (A) and oral mucosa (B) of rats by qRT–PCR as described in Materials and Methods. Only genes that showed significant alterations are included and the values represent mean fold change ± SD (n =6). Open bars and shaded bars represent controls and (S)-NNN-treated rats, respectively. Statistical significance was assessed relative to the vehicle control group using one–way ANOVA and a post Tukey’s test; *p < 0.05. (C) Validation of EPHX2 alteration in RNA sequencing by Western immunoblot analysis. The assays were performed three times as described in the Materials and Methods section. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The experiment was done three times. Statistical significance was assessed relative to the vehicle control group using one–way ANOVA and a post Tukey’s test; *p < 0.05
In line with the mRNA results, immunoblotting analysis showed increased levels of soluble epoxide hydrolase (Ephx2) in esophageal mucosa of (S)-NNN-treated rats; although not significant, a similar trend was observed in oral cells (Figure 3C).
Exposure of OKF-6 oral keratinocytes to (S)-NNN and (R)-NNN alters the expression of the same genes deregulated in esophageal and oral mucosa of rats exposed to (S)-NNN
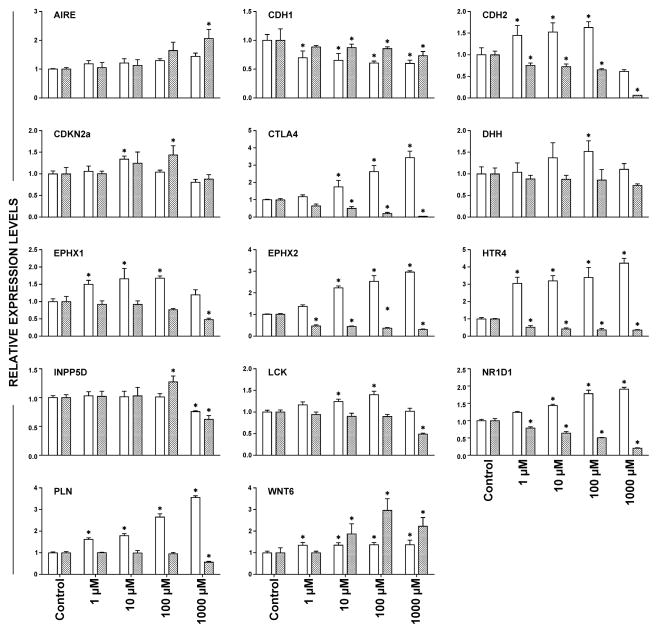
To determine if a common signature existed between our experimental model and human cell lines upon carcinogen treatment, we exposed immortalized oral keratinocytes (OKF6 cells) to (S)-NNN and the opposite enantiomer (R)-NNN, and the levels of 14 genes were analyzed by qRT-PCR in treated versus untreated cells. As shown in Figure 4, (S)-NNN modulated most of the genes significantly. The changes in the expression of microsomal epoxide hydrolase (EPHX1), soluble epoxide hydrolase (EPHX2), 5-hydroxytryptamine (serotonin) receptor 4 (HTR4), phospholamban (PLN), cytotoxic T lymphocyte-associated molecule-4 (CTLA4), and lymphocyte-specific protein tyrosine kinase (LCK) were consistent and dose-dependent. Exposure of OKF6 cells to (R)-NNN up-regulated the level of WNT6, but downregulated the expression of HTR4, EPHX2, and CTLA4. Since WNT6 and DHH are genes that regulate stemness and epithelial mesenchymal transition (EMT), and these genes were altered by the carcinogens, we examined the effect of (S)-NNN and (R)-NNN on E-cadherin (CDH1) and N-cadherin (CDH2). E-cadherin and N-cadherin are reduced and increased, respectively, during EMT. As shown in Figure 4, (S)-NNN induced a switch from E-cadherin to N-cadherin, but the effect of (R)-NNN was unclear.
Figure 4.
Gene expression alterations induced by (S)-NNN and (R)-NNN in OKF6 immortalized oral keratinocytes. Selected genes from those deregulated by (S)-NNN in esophageal and oral mucosa of rats were analyzed in RNA samples obtained from (S)-NNN-treated (unfilled bars) or (R)-NNN-treated (filled bars) OKF6 cells by qRT-PCR as described in Materials and Methods. Expression levels are shown in arbitrary units on the Y axis, whereas concentrations of the carcinogens are shown on the X axis. Statistical significance was assessed relative to the level of the genes in untreated OKF6 cells using one–way ANOVA and a post Tukey’s test; *P < 0.05
Genes deregulated by (S)-NNN in rat oral and esophageal mucosa show altered expression in premalignant oral leukoplakia cells and oral and head and neck carcinoma cells
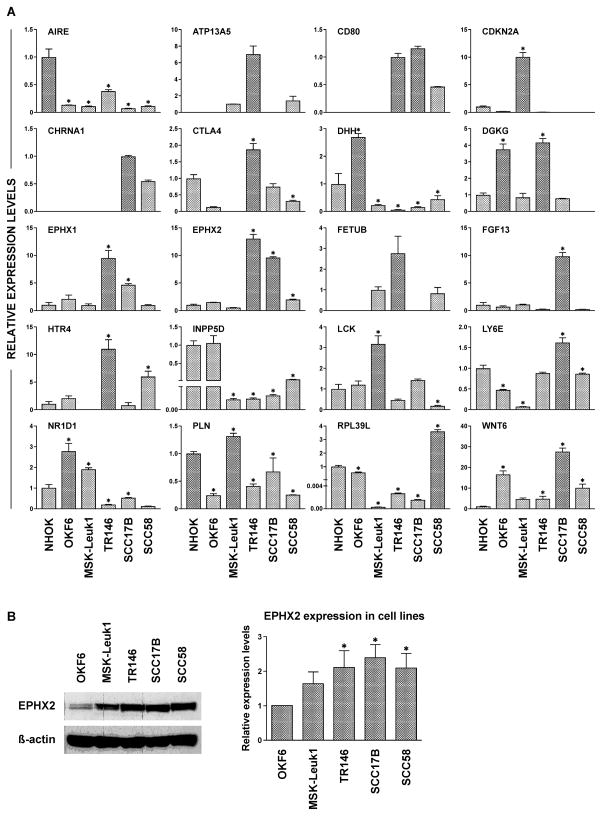
To assess if the genes deregulated by (S)-NNN in oral and esophageal mucosa of rats are temporally regulated during human oral carcinogenesis, we compared the level of these genes in immortalized oral keratinocytes, premalignant oral leukoplakia cells and oral and head and neck cancer cell lines. Generally, the results were highly variable among the different cell lines, including the two immortalized cell lines. However, ATP13A5, CD80, CHRNA1 and FETUB were consistently undetected in normal immortalized cells, whereas the expressions of LCK and CDKN2A were significantly higher in premalignant Msk-Leuk1 cells than the levels of these genes in immortalized or malignant cells. Moreover, expressions of DHH and INPP5D were consistently downregulated in the premalignant line as well as all three malignant cell lines. Among the three cancer cell lines, most genes were upregulated in TR146 cells (ATP13A5, CTLA4, DGKG, EPHX1, EPHX2, FETUB, and HTR4).
To determine if the differential expression of soluble epoxide hydrolase (EPHX2) gene in cells at different stages of transformation would be paralleled by similar alterations at a protein level, we analyzed the expression of the protein in the four cell lines by immunoblotting. As depicted in Figure 5B, the level of EPHX2 significantly increased, compared to the expression in immortalized OKF6 cells, by about 50% in premalignant Msk-Leuk1 cells and by 2-fold or more in the cancer cells.
Figure 5.
Genes deregulated by (S)-NNN in esophageal and oral mucosa of rats are differentially expressed in oral and head neck cells at different stages of transformation. (A) Selected genes from those deregulated by (S)-NNN in esophageal and oral mucosa of rats were analyzed in RNA samples from NHOK, OKF6, Msk-Leuk1, TR146, SCC17B and SCC58 cells by qRT-PCR as described in the Materials and Methods section. Expression levels are shown in arbitrary units on the Y axis, whereas the different cell lines are shown on the X axis. (B) Validation of EPHX2 alteration in the different cell lines by Western immunoblot analysis. The assays were performed three times as described in Materials and Methods and the results are presented as mean ± SD. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. Statistical significance was assessed relative to level of the protein in OKF6 cells using one–way ANOVA and a post Tukey’s test; *p < 0.05
Discussion
The objective of this study was to understand molecular mechanisms underlying (S)-NNN-induced oral and esophageal tumorigenesis, and identify genes and pathways that may facilitate early detection and chemoprevention strategies. Overall, exposure of rats to (S)-NNN led to perturbations in genes involved in immune regulation, cell cycle, cell signaling, epithelial-mesenchymal transition, and carcinogen metabolism in both esophageal and oral tissues. However, gene expression deregulations in oral tissues were weaker and more inconsistent than in esophageal tissues, probably related to the sample collection method (aliquots of tissues were sampled from different areas of the oral cavity which might be differentially exposed to the carcinogen or have different biological responses to carcinogen exposure). The results in rat tissues were further corroborated in (S)-NNN-treated immortalized oral keratinocytes, oral and head and neck cell lines at different stages of transformation (immortalized, premalignant and malignant) and publicly available human esophageal carcinoma and head and neck squamous cell carcinoma TCGA data sets.
Recent studies in several cancer models consistently indicate that an immunosuppressive environment is established early in tumor development to effectively thwart the immune response to neoplastic cells [24–27]. Therefore, immune-prevention is now considered as a strategy to prevent cancer through the use of vaccines, antibodies, and immune modulators [28]. In this study, autoimmune regulator (Aire), the central regulator of immune tolerance, and cytotoxic T lymphocyte-associated molecule-4 (Ctla-4), a powerful negative regulator of T cell activation, were upregulated in both oral and esophageal tissues of (S)-NNN-treated rats. Similar trends were observed in studies with oral and head and neck cancer cell lines. Aire is believed to induce immunosuppression via clonal deletion of self-reactive thymocytes and the development of immune-suppressive regulatory T cells [29, 30]. Aire deficient mice displayed elevated T cell immune responses that were associated with suppression of melanoma outgrowth and transplantation of Aire-deficient thymic stroma was sufficient to confer more effective immune rejection of melanoma in an otherwise Aire wild type host [30]. Similarly, Ctla-4 inhibits T cell activation by competing with CD28, a protein that provides co-stimulatory signals required for T cell activation and survival, for binding to leucocyte surface molecules CD80 and CD86, and thereby dampening of T-cell activation and proliferation [31, 32]. Ipilimumab, an antibody directed against Ctla-4, is approved by FDA for the treatment of melanoma and it is undergoing clinical trials for the treatment of several cancers, including non-small cell lung carcinoma, small cell lung cancer, bladder cancer, and metastatic hormone-refractory prostate cancer [31, 32]. No previous reports are available on the modulation of Aire or Ctla-4 by tobacco smoke or tobacco smoke carcinogens. However, NNK downregulated T-cell proliferative responses to mitogens and antigens and impaired an antigen-mediated T-cell signaling pathway in A/J mice and these effects were postulated to be associated with NNK-induced expression of α7-nAChRs [25].
Cancer-associated genes significantly up-regulated in esophageal and, to a lesser extent, in oral tissues of (S)-NNN-treated rats include cyclin-dependent kinase inhibitor 2A (Cdkn2a, P16), epoxide hydrolase (Ephx1, Ephx2), desert hedgehog (Dhh), 5-hydroxytryptamine receptor 4 (HTR4), inositol polyphosphate-5-phosphatase (Inpp5d), lymphocyte-specific protein tyrosine kinase (Lck), nuclear receptor subfamily 1, group D, member 1 (Nr1d1), phopsholamban (Pln), Wnt6, and Fetub. Most of these genes were also upregulated in (S)-NNN-treated OKF6 immortalized oral keratinocytes and premalignant Msk-Leuk1 oral cells, corroborating the results from the rat studies that these genes are deregulated during the early stage of oral and esophageal carcinogenesis.
CDKN2A, the principal member of the Ink4 family of cyclin-dependent kinase inhibitors, is a tumor suppressor gene frequently down-regulated in a large number of tumors [33,34]. However, in line with our results in esophageal and oral mucosa from (S)-NNN-treated rats, premalignant oral cells and (S)-NNN-treated immortalized oral keratinocytes exhibited upregulation of CDKN2A. Overexpression of CDKN2A has been reported in several benign and pre-malignant lesions [35] and this phenomenon is believed to control cell proliferation in response to oncogenic stimuli, thereby protecting cells from malignant transformation. Thus, CDKN2A overexpression could have a diagnostic application to differentiate between premalignant lesions and carcinoma [35].
sEH is a cytosolic enzyme with epoxide hydrolase and lipid phosphatase activities that catalyzes the rapid hydrolysis of anti-inflammatory epoxyeicosatrienoic acids (EETs) to their inactive or less active 1,2-diols [36]. Therefore, inhibition of sEH could suppress chronic inflammation-associated cancers. Indeed, in one recent study, genetic knockout of sEH inhibited dextran sulfate sodium-induced colitis, inflammatory cytokines and chemokines, pre-cancerous dysplastic lesions and colon tumor incidence and tumor size [37]. In the present study, both forms of epoxide hydrolase (Ephx1 and Ephx2) increased in esophageal tissues of (S)-NNN-treated rats and (S)-NNN-treated immortalized oral keratinocytes and oral and head and neck cancer cell lines. Ephx1 plays a critical role in the metabolism of numerous xenobiotic compounds including the bioactivation of polycyclic aromatic hydrocarbon carcinogens [38]. Immunoblotting studies with rat esophageal tissues and immortalized, premalignant and malignant oral cells showed that EPHX2 is overexpressed in premalignant cells and the level further increased in cancer cells. Thus, it appears that induction of sEH plays a role in the promotion and progression of inflammation-associated cancers such as oral and esophageal cancer and thus could serve as a biomarker for the early detection of these diseases and as a target for chemopreventive and therapeutic agents.
Tobacco smoke carcinogen-induced alterations in stem cell signaling pathways and EMT genes have a role in cancer initiation and clonal expansion of premalignant lung epithelial cells [39]. Consistent with this, esophageal tissues from (S)-NNN-treated rats as well as (S)-NNN-and (R)-NNN-treated immortalized oral keratinocytes exhibited overexpression of WNT6 and DHH, which are known to regulate the proliferation of normal and cancer stem cells and acquisition of epithelial to mesenchymal transition [40, 41]. Furthermore, exposure of immortalized oral cells to (S)-NNN induced downregulation and upregulation of E-cadherin (CDH1) and N-cadherin (CDH2), respectively, a phenomenon considered to be the main characteristic feature of EMT [42].
Although their role in carcinogenesis has not been thoroughly investigated, LCK, PLN and HTR4 exhibited consistent overexpression in oral and esophageal tissues of (S)-NNN-treated rats, (S)-NNN treated oral keratinocytes and untreated premalignant Msk-Leuk 1 cells. LCK is a member of the Src family nonreceptor protein-tyrosine kinase and aberrant LCK expression and kinase activity have been implicated in the pathogenesis of both lymphoid and nonlymphoid malignancies [43]. The role of PLN in carcinogenesis is unclear, but it plays an important role in calcium homeostasis of the heart muscle by regulating Ca++ -uptake by the cardiac sarcoplasmic reticulum. HTR4 is a member of 7 different families of serotonin receptors and is believed to contribute to carcinogenesis by stimulating growth factors [44].
Interrogation of publicly available human esophageal carcinoma and HNSCC TCGA data sets showed that some of the genes deregulated in the rat tissues also exhibited altered gene copy numbers and expression levels in human esophageal and HNSCC tumor samples. Interestingly, only some but not all genes with genetic alternations showed changes in mRNA expression. Among genes overexpressed in the esophagus of (S)-NNN-treated rats, ATP13A5, fetulin B (FETUB) and lymphocyte antigen 6 complex locus E (LY6E) genes were amplified in 23%, 20% and 14%, respectively, of human esophageal tumors. In addition to copy number amplification, both ATP13A5 and LY6E showed mRNA upregulation in human ESCA, suggesting their potential roles in early onset and maintenance of tumorigenesis as a result of increases in genetic activities. The role of ATP13A5, a member of P5-ATPases which are involved in the transport of inorganic cations and other substrates across cell membranes [45], in cancer is unknown, whereas FETUB, a member of the cystatin protein family, is overexpressed in human high grade esophageal dysplastic lesions and adenocarcinoma [46]. LY6E is a negative immune regulator of monocytes, known to be overexpressed in several cancers, including ovarian, pancreatic, lung, gastric, and various breast cancers [47]. Among genes overexpressed in the oral mucosa of (S)-NNN-treated rats, diacylglycerol kinase gamma (DGKG) is the most commonly amplified (in 30% of cases) and overexpressed gene in HNSCC. Its biological function is to catalyze the phosphorylation of diacylglycerol, a fundamental lipid second messenger that activates numerous proteins, including protein kinase C isoforms, Ras guanyl nucleotide-releasing proteins and transient receptor potential channels [48]. Certain genes were downregulated in rat tissues but showed amplification of gene copy number in the TCGA data. For instance, ribosomal protein L39 (RPL39L), which is implicated in tumor growth progression, and metastasis [49–51] was downregulated in esophageal mucosa of (S)-NNN-treated rats, but exhibited copy number amplification in 21% of cases and mRNA upregulation (data not shown) in esophageal tumors from TCGA data, indicating dual roles depending on the stage of carcinogenesis.
In summary, our data indicate that immunoregulatory and cancer-associated genes are the most deregulated genes during the early stage of (S)-NNN-induced oral and esophageal carcinogenesis. Studies with cell lines at different stages of transformation indicated that most of these genes remain altered during premalignant and malignant stages of tumorigenesis as well. Therefore, these genes could be used as biomarkers for the early detection of oral and esophageal cancer and as targets for chemoprevention.
Supplementary Material
Analysis of genes upregulated by (S)-NNN in the human TCGA data. (A) OncoPrint of genes upregulated by (S)-NNN (0.005 < p < 0.05) in rat esophagus showing CNA and/or deep deletion in human ESCA. (B) OncoPrint of genes upregulated by (S)-NNN (0.005 < p < 0.05) in rat oral cavity and showed CNA, deep deletion, mRNA downregulation/upregulation, missense mutation and/or truncating mutation in human HNSCC.
Table 2.
Genes deregulated by (S)-NNN in rat oral cavity tissues are also altered in human head and neck squamous cell carcinoma (HNSCC) using TCGAa.
| Rat Oral Cavity RNA-seq | Human TCGA HNSCC RNA-Seq | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Rat gene ID | Human Symbol | Fold Change | p-value | Fold Change | p-value | FDR |
| Gip | GIP | 19.1 | 0.00401 | 9.0 | 9.63E-14 | 1.84E-13 |
| Ano3 | ANO3 | 11.8 | 0.00713 | 2.1 | 1.08E-05 | 1.16E-05 |
| Aire | AIRE | 11.6 | 0.0212 | 2.4 | 2.22E-06 | 2.52E-06 |
| Apba2 | APBA2 | 5.2 | 0.00159 | 6.3 | 5.52E-24 | 1.67E-23 |
| Gria3 | GRIA3 | 4.2 | 1.02E-07 | 4.8 | 1.88E-18 | 4.59E-18 |
| Pou2f2 | POU2F2 | 4.1 | 0.0477 | 2.3 | 2.71E-07 | 3.27E-07 |
| Cd80 | CD80 | 3.9 | 0.000908 | 4.8 | 1.84E-18 | 4.49E-18 |
| Spta1 | SPTA1 | 3.5 | 0.00929 | 3.3 | 1.62E-10 | 2.49E-10 |
| Dgkg | DGKG | 3.1 | 0.0008 | 2.5 | 1.51E-08 | 2.01E-08 |
| Gdf6 | GDF6 | 2.9 | 0.0194 | 5.8 | 7.89E-21 | 2.12E-20 |
| Kcnb2 | KCNB2 | 2.9 | 0.0373 | 2.0 | 1.68E-05 | 1.79E-05 |
| Tfpi2 | TFPI2 | 2.8 | 0.0482 | 6.5 | 1.77E-24 | 5.47E-24 |
| Rsad2 | RSAD2 | 2.4 | 0.0172 | 5.6 | 9.79E-22 | 2.74E-21 |
| Itga2b | ITGA2B | 2.2 | 0.0452 | 3.0 | 2.92E-10 | 4.41E-10 |
| Lsamp | LSAMP | 2.2 | 0.0272 | 3.1 | 1.20E-11 | 2.00E-11 |
| RGD1559864 | KIAA1045 | 2.2 | 0.0382 | 4.8 | 6.70E-19 | 1.67E-18 |
| Tbx18 | TBX18 | 2.2 | 0.00482 | 3.2 | 4.77E-12 | 8.20E-12 |
| Slc16a7 | SLC16A7 | −2.1 | 0.00191 | −4.3 | 6.99E-36 | 2.93E-35 |
| Gjb1 | GJB1 | −2.6 | 0.000908 | −15.3 | 1.04E-157 | 1.77E-156 |
| Rhcg | RHCG | −2.6 | 0.0368 | −7.6 | 1.93E-78 | 1.59E-77 |
| Dcdc2 | DCDC2 | −3.3 | 0.00328 | −2.3 | 1.38E-10 | 2.14E-10 |
| Cyp2b1 | CYP2B6 | −3.6 | 0.0159 | −7.7 | 2.21E-66 | 1.56E-65 |
| Gnat3 | GNAT3 | −4.6 | 0.039 | −3.0 | 1.05E-09 | 1.53E-09 |
| Sgk2 | SGK2 | −6.3 | 0.0221 | −6.7 | 4.75E-65 | 3.30E-64 |
| Chia | CHIA | −6.7 | 0.00186 | −2.3 | 5.49E-06 | 6.05E-06 |
| Myh8 | MYH8 | −7.2 | 0.0183 | −3.0 | 2.92E-19 | 7.37E-19 |
| Hnf1b | HNF1B | −155.9 | 0.00772 | −8.9 | 1.32E-74 | 1.04E-73 |
Fold changes represent changes in (or ratio of) mRNA expression levels in comparison to untreated animals or normal adjacent tissues. Negative fold changes represent downregulation in mRNA expression at the indicated ratios. FDR, false discovery rate.
Acknowledgments
This study was supported by grant CA-81301 from the National Cancer Institute (to SSH) and Faculty Start-up from Masonic Cancer Center and College of Veterinary Medicine (to FK). We are indebted to Bob Carlson for his editorial assistance.
Definitions for all abbreviations used
- CNA
Copy number alternations
- DMEM-F12
Dulbecco’s Modified Eagle Medium/Ham’s F-12
- EET
Epoxyeicosatrienoic acids
- EMT
Epithelial mesenchymal transition
- ESCA
Esophageal carcinoma
- GISTIC
Genomic Identification of Significant Targets in Cancer
- HNSCC
Head and neck squamous cell carcinoma
- hTERT
human telomerase reverse transcriptase
- IARC
the International Agency for Research on Cancer
- KSFM
Keratinocyte serum free media
- NNK
Nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- POB
Pyridyloxobutyl
- qRT-PCR
Quantitative real time polymerase chain reaction
- RSEM
RNA-Seq by expectation-maximization
- SCC
Squamous cell carcinoma
- SD
Standard deviation
- SEM
Standard error mean
- Seq
Sequencing
- STR
Short tandem repeat
- TCGA
The cancer genome atlas
- TMM
Trimmed Mean of M-values
References
- 1.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 2.Balbo S, James-Yi S, Johnson CS, et al. (S)-N′-Nitrosonornicotine, a constituent of smokeless tobacco, is a powerful oral cavity carcinogen in rats. Carcinogenesis. 2013;34:2178–2183. doi: 10.1093/carcin/bgt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hecht SS, Rivenson A, Braley J, DiBello J, Adams JD, Hoffmann D. Induction of oral cavity tumors in F344 rats by tobacco-specific nitrosamines and snuff. Cancer Res. 1986;46:4162–4166. [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 89. Lyon, France: Smokeless tobacco and tobacco-specific nitrosamines; 2007. [PMC free article] [PubMed] [Google Scholar]
- 5.Carmella SG, McIntee EJ, Chen M, Hecht SS. Enantiomeric composition of N′-nitrosonornicotine and N′-nitrosoanatabine in tobacco. Carcinogenesis. 2000;21:839–843. doi: 10.1093/carcin/21.4.839. [DOI] [PubMed] [Google Scholar]
- 6.Stepanov I, Biener L, Knezevich A, et al. Monitoring tobacco-specific N-nitrosamines and nicotine in novel Marlboro and Camel smokeless tobacco products: findings from round 1 of the New Product Watch. Nicotine Tob Res. 2011;14:274–281. doi: 10.1093/ntr/ntr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stepanov I, Yershova K, Carmella S, Upadhyaya P, Hecht SS. Levels of (S)-N′-nitrosonornicotine in U.S. tobacco products. Nicotine Tob Res. 2013;15:1305–1310. doi: 10.1093/ntr/nts249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIntee EJ, Hecht SS. Metabolism of N′-nitrosonornicotine enantiomers by cultured rat esophagus and in vivo in rats. Chem Res Toxicol. 2000;13:192–199. doi: 10.1021/tx990171l. [DOI] [PubMed] [Google Scholar]
- 9.Lao Y, Yu N, Kassie F, Villalta PW, Hecht SS. Analysis of pyridyloxobutyl DNA adducts in F344 rats chronically treated with (R)- and (S)-N′-nitrosonornicotine. Chem Res Toxico. 2007;20:246–256. doi: 10.1021/tx060208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S, Wang M, Villalta PW, Lindgren BR, Lao Y, Hecht SS. Quantitation of pyridyloxobutyl DNA adducts in nasal and oral mucosa of rats treated chronically with enantiomers of N′-nitrosonornicotine. Chem Res Toxicol. 2009;22:949–956. doi: 10.1021/tx900040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeman JI, Chavdarian CG, Secor HV. Synthesis of the enantiomers of nornicotine. J Org Chem. 1997;50:5419–5421. [Google Scholar]
- 12.Kim RH, Kang RH, Shin KH, et al. Bmi-1 cooperates with human papillomavirus type 16 E6 to immortalize normal human oral keratinocytes. Exp Cell Res. 313(3):462–72. doi: 10.1016/j.yexcr.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Dickson MA, Hahn WC, Ino Y, et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gümüş ZH, Du B, Kacker A, et al. Effects of tobacco smoke on gene expression and cellular pathways in a cellular model of oral leukoplakia. Cancer Prev Res. 2008;1:100–111. doi: 10.1158/1940-6207.CAPR-08-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian X, Khammanivong A, Song JM, et al. RNA-sequencing studies identify genes differentially regulated during inflammation-driven lung tumorigenesis and targeted by chemopreventive agents. Inflamm Res. 2015;64:343–361. doi: 10.1007/s00011-015-0815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lie B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;2:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beroukhim R, Getz G, Nghiemphu L, et al. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci U S A. 2007;104:20007–20012. doi: 10.1073/pnas.0710052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song JM, Qian X, Molla K, et al. Combinations of indole-3-carbinol and silibinin suppress inflammation-driven mouse lung tumorigenesis by modulating critical cell cycle regulators. Carcinogenesis. 2015;36:666–675. doi: 10.1093/carcin/bgv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998;8:186–194. [PubMed] [Google Scholar]
- 24.Inman KS, Francis AA, Murray NR. Complex role for the immune system in initiation and progression of pancreatic cancer. World J Gastroenterol. 2014;20:11160–11181. doi: 10.3748/wjg.v20.i32.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razani-Boroujerdi S, Sopori ML. Early manifestations of NNK-induced lung cancer: role of lung immunity in tumor susceptibility. Am J Respir Cell Mol Biol. 2007;36:13–19. doi: 10.1165/rcmb.2005-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holtzhausen A, Zhao F, Evans KS, Hanks BA. Early Carcinogenesis Involves the Establishment of Immune Privilege via Intrinsic and Extrinsic Regulation of Indoleamine 2,3-dioxygenase-1: Translational Implications in Cancer Immunotherapy. Front Immunol. 2014;5:438. doi: 10.3389/fimmu.2014.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasti TH, Rudemiller KJ, Cochran JB, et al. Immunoprevention of chemical carcinogenesis through early recognition of oncogene mutations. J Immunol. 2015;194:2683–2695. doi: 10.4049/jimmunol.1402125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marzbani E, Inatsuka C, Lu H, Disis ML. The invisible arm of immunity in common cancer chemoprevention agents. Cancer Prev Res. 2013;6:764–773. doi: 10.1158/1940-6207.CAPR-13-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peter A, Leventhal DS, Malchow S. Shaping the repertoire of tumor-infiltrating effector and regulatory T cells. Immunol Rev. 2014;259:245–258. doi: 10.1111/imr.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu M, Nagavalli A, Su MA. Aire deficiency promotes TRP-1 specific immune rejection of melanoma. Cancer Res. 2013;73:2104–2116. doi: 10.1158/0008-5472.CAN-12-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camacho LH. CTLA-4 blockade with ipilimumab: biology, safety, efficacy, and future considerations. Cancer Med. 2015;4:661–672. doi: 10.1002/cam4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honeychurch J, Cheadle EJ, Dovedi SJ, Illidge TM. Immuno-regulatory antibodies for the treatment of cancer. Expert Opin Biol Ther. 2015;15:787–801. doi: 10.1517/14712598.2015.1036737. [DOI] [PubMed] [Google Scholar]
- 33.Liggett WH, Sidransky D. Role of the p16 tumor suppressor gene in cancer. J Clin Oncol. 1998;16:1197–1206. doi: 10.1200/JCO.1998.16.3.1197. [DOI] [PubMed] [Google Scholar]
- 34.Romagosa C, Simonetti S, López-Vicente L, et al. p16Ink4a overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene. 2011;30:2087–2097. doi: 10.1038/onc.2010.614. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Rosen DG, Yao JL, Huang J, Liu J. Expression of p14ARF, p15INK4b, p16INK4a, and DCR2 increases during prostate cancer progression. Mod Pathol. 2006;19:1339–1343. doi: 10.1038/modpathol.3800655. [DOI] [PubMed] [Google Scholar]
- 36.Morisseau C, Hammock BD. Impact of Soluble Epoxide Hydrolase and Epoxyeicosanoids on Human Health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W, Liao J, Li H, et al. Reduction of inflammatory bowel disease-induced tumor development in IL-10 knockout mice with soluble epoxide hydrolase gene deficiency. Mol Carcinog. 2013;52:726–738. doi: 10.1002/mc.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fretland AJ, Omiecinski CJ. Epoxide hydrolases: biochemistry and molecular biology. Chem Biol Interact. 2000;129:41–59. doi: 10.1016/s0009-2797(00)00197-6. [DOI] [PubMed] [Google Scholar]
- 39.Tellez CS, Juri DE, Do K, et al. EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells. Cancer Res. 2011;71:3087–3097. doi: 10.1158/0008-5472.CAN-10-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan G, Regel I, Lian F, et al. WNT6 is a novel target gene of caveolin-1 promoting chemoresistance to epirubicin in human gastric cancer cells. Oncogene. 2013;32:375–387. doi: 10.1038/onc.2012.40. [DOI] [PubMed] [Google Scholar]
- 41.Kayed H, Kleeff J, Osman T, Keleg S, Büchler MW, Friess H. Hedgehog signaling in the normal and diseased pancreas. Pancreas. 2006;32:119–129. doi: 10.1097/01.mpa.0000202937.55460.0c. [DOI] [PubMed] [Google Scholar]
- 42.Yuki K, Yoshida Y, Inagaki R, Hiai H, Noda M. E-cadherin-downregulation and RECK-upregulation are coupled in the non-malignant epithelial cell line MCF10A but not in multiple carcinoma-derived cell lines. Sci Rep. 2014;4:4568. doi: 10.1038/srep04568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majolini MB, Boncristiano M, Baldari CT. Dysregulation of the protein tyrosine kinase Lck in lymphoproliferative disorders and in other neoplasias. Leuk Lymphoma. 1999;35:245–254. doi: 10.3109/10428199909145727. [DOI] [PubMed] [Google Scholar]
- 44.Kopparapu PK, Tinzl M, Anagnostaki L, Persson JL, Dizeyi N. Expression and localization of serotonin receptors in human breast cancer. Anticancer Res. 2013;33:363–370. [PubMed] [Google Scholar]
- 45.Schultheis PJ, Hagen TT, O’Toole KK, et al. Characterization of the P5 subfamily of P-type transport ATPases in mice. Biochem Biophys Res Commun. 2004;323:731–738. doi: 10.1016/j.bbrc.2004.08.156. [DOI] [PubMed] [Google Scholar]
- 46.Mann B, Madera M, Klouckova, et al. A quantitative investigation of fucosylated serum glycoproteins with application to esophageal adenocarcinoma. Electrophoresis. 2010;31:1833–1841. doi: 10.1002/elps.201000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asundi J, Crocker L, Tremayne J, et al. An Antibody-Drug Conjugate Directed against Lymphocyte Antigen 6 Complex, Locus E (LY6E) Provides Robust Tumor Killing in a Wide Range of Solid Tumor Malignancies. Clin Cancer Res. 2015;21:3252–3262. doi: 10.1158/1078-0432.CCR-15-0156. [DOI] [PubMed] [Google Scholar]
- 48.Kai M, Sakane F, Imai S, Wada I, Kanoh H. Molecular cloning of a diacylglycerol kinase isozyme predominantly expressed in human retina with a truncated and inactive enzyme expression in most other human cells. J Biol Chem. 1994;269:18492–18498. [PubMed] [Google Scholar]
- 49.Dave B, Granados-Principal S, Zhu R, et al. Targeting RPL39 and MLF2 reduces tumor initiation and metastasis in breast cancer by inhibiting nitric oxide synthase signaling. Proc Natl Acad Sci U S A. 2014;111:8838–8843. doi: 10.1073/pnas.1320769111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray S, Johnston R, Campbell DC, et al. Androgens and estrogens stimulate ribosome biogenesis in prostate and breast cancer cells in receptor dependent manner. Gene. 2013;526:46–53. doi: 10.1016/j.gene.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 51.Gandin V, Miluzio A, Barbieri AM, et al. Eukaryotic initiation factor 6 is rate-limiting in translation, growth and transformation. Nature. 2008;455:684–688. doi: 10.1038/nature07267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of genes upregulated by (S)-NNN in the human TCGA data. (A) OncoPrint of genes upregulated by (S)-NNN (0.005 < p < 0.05) in rat esophagus showing CNA and/or deep deletion in human ESCA. (B) OncoPrint of genes upregulated by (S)-NNN (0.005 < p < 0.05) in rat oral cavity and showed CNA, deep deletion, mRNA downregulation/upregulation, missense mutation and/or truncating mutation in human HNSCC.