Abstract
Accurate identification of Arctic plant species is critical for understanding potential climate-induced changes in their diversity and distributions. To facilitate rapid identification we generated DNA barcodes for the core plastid barcode loci (rbcL and matK) for 490 vascular plant species, representing nearly half of the Canadian Arctic flora and 93% of the flora of the Canadian Arctic Archipelago. Sequence recovery was higher for rbcL than matK (93% and 81%), and rbcL was easier to recover than matK from herbarium specimens (92% and 77%). Distance-based and sequence-similarity analyses of combined rbcL + matK data discriminate 97% of genera, 56% of species, and 7% of infraspecific taxa. There is a significant negative correlation between the number of species sampled per genus and the percent species resolution per genus. We characterize barcode variation in detail in the ten largest genera sampled (Carex, Draba, Festuca, Pedicularis, Poa, Potentilla, Puccinellia, Ranunculus, Salix, and Saxifraga) in the context of their phylogenetic relationships and taxonomy. Discrimination with the core barcode loci in these genera ranges from 0% in Salix to 85% in Carex. Haplotype variation in multiple genera does not correspond to species boundaries, including Taraxacum, in which the distribution of plastid haplotypes among Arctic species is consistent with plastid variation documented in non-Arctic species. Introgression of Poa glauca plastid DNA into multiple individuals of P. hartzii is problematic for identification of these species with DNA barcodes. Of three supplementary barcode loci (psbA–trnH, psbK–psbI, atpF–atpH) collected for a subset of Poa and Puccinellia species, only atpF–atpH improved discrimination in Puccinellia, compared with rbcL and matK. Variation in matK in Vaccinium uliginosum and rbcL in Saxifraga oppositifolia corresponds to variation in other loci used to characterize the phylogeographic histories of these Arctic-alpine species.
Introduction
Arctic regions of the world are changing rapidly in response to climate change. Understanding the diversity and distributions of Arctic plant species in the past and present is critical for documenting change, and accurate identification of plant species underpins all research on Arctic vegetation. Changes in Arctic vegetation linked to climate change include increased plant productivity [1–6], shrub expansion [7–11], changes in community composition (species diversity and abundance) of varying degree [1,4,12–15], and treeline advancement [16].
The Arctic ecozone in Canada extends from northwestern Yukon to northern Newfoundland and Labrador and represents 36% of the global Arctic [17,18]. The Canadian Arctic flora has been studied since explorers searched for the Northwest Passage in the early 1800s (e.g., [19]), but knowledge of its composition, distribution, and evolutionary history remains incomplete. There are several Arctic genera in which species boundaries are not clear and/or species are difficult to identify [20]. Authoritative floras and checklists synthesizing knowledge of Canadian Arctic plants have been published [20–25] and there is an extensive literature reporting floristic discoveries in the Arctic (e.g., [26–30]), but there is no vascular plant flora covering the entire Canadian Arctic ecozone. The number of vascular plant species in this large region is not known with precision. The Panarctic Flora divides the global Arctic—defined fairly broadly in North America to include regions with numerous borderline Arctic taxa in the Brooks Range, along the Mackenzie River Delta, near Great Bear Lake, and along Hudson Bay—into biogeographical zones, and provides data on the distribution and frequency of taxa in each zone [20]. The North American Arctic is divided into six zones: Western Alaska, Northern Alaska–Yukon Territory, Central Canada, Hudson Bay–Labrador, Ellesmere Land–Northern Greenland, and Western Greenland. There are some 1100 vascular plant species in the four zones that include Canada [20], but the number of species restricted to Canada is probably lower, as there are species in the trans-national zones that do not occur in Canada. In the Canadian Arctic Archipelago, a more easily defined geographical region, there are 341 vascular plant species (349 taxa) [25].
DNA Barcoding
DNA barcoding is the use of short regions of DNA—the DNA barcode—to identify species by assigning individuals to known taxa through comparison of their barcodes with a reference library. The search for a plant DNA barcode has been challenging. The mitochondrial locus cytochrome oxidase I (COI) widely used as a DNA barcode for animal taxa (e.g., [31–33]) is not suitable as a plant barcode due to its (usually) low variability—a function of the (usually) low substitution rate of the mitochondrial genome in plants compared with animals [34–37] and substantial variation in plant mitochondrial genome structure [38]. Multiple coding and non-coding plastid loci, alone or combined, have been proposed and tested as potential plant DNA barcodes (e.g., [34,37,39–50], reviewed in Hollingsworth et al. [51]). A comparison of the performances of eight of these regions demonstrated that (1) multi-locus plant barcodes perform better than single-locus barcodes, (2) various combinations of plastid regions performed similarly, and (3) a maximum of 71% of species could be distinguished with plastid barcodes [34]. The Consortium for the Barcode of Life (CBOL) Plant Working Group [52] conducted a broader study of the performance of candidate barcode regions (atpF–atpH, matK, rbcL, rpoB, rpoC1, psbK–psbI, psbA–trnH), and recommended rbcL + matK as the standard or core plant barcode. Recognizing that these two loci discriminate only some 70% of plant species, they acknowledged that supplementary loci will be required to improve identification success in many plant groups. The Executive Committee of the Consortium for the Barcode of Life (2009) approved matK and rbcL as the required core barcode regions for land plants, and also encouraged researchers to collect data from supplementary non-coding loci [53].
The nuclear ribosomal internal transcribed spacer regions (ITS1 and ITS2) were also initially considered as possible plant barcode regions (e.g., [37,39,54]), but dismissed due to complicating problems, including fungal contamination, paralogous copies, and amplification difficulties [51,55]. Despite these limitations, recent studies have demonstrated substantially higher discrimination rates for one or both ITS regions compared with plastid barcodes [56–58]. The largest of these was conducted by the China Plant BOL Group [58], who tested the performance of four barcoding regions (rbcL, matK, psbA–trnH, ITS) in a large data set (6,286 individuals, 1,757 species), and found ITS alone had the highest discriminatory power. There is renewed interest in ITS as an additional core plant barcode region [55,58], and many plant barcoding studies are now including ITS along with the core and/or other plastid loci.
There has been an explosion of plant DNA barcoding studies in recent years that test the performance of candidate DNA barcode regions and recommend markers best suited to particular taxa. Many have focused on individual or closely related genera [59–73], families [44,74–83], and higher taxa [84–87]. Studies that sample multiple closely related species generally have the lowest species resolution among barcoding studies, whereas studies that do not sample multiple close relatives generally have the highest species discrimination [51]. The latter includes studies of non-taxonomic groups such as medicinal plants [57,88,89], crop species [90,91], and invasive species [92,93]. Another subset of non-taxonomic studies has focused on barcoding the floras or subsets of the floras of restricted geographical areas, in which diversity is lower and fewer closely related species are expected compared with floras of broader geographical areas. Species identification with DNA barcodes in some of these studies has been high: >98% for forest plots on Barro Colorado Island in Panama [94]; >90% for orchids of Mesoamerica and trees, shrubs and achlorophyllous parasites from Kruger National Park, South Africa [43]; >93% for the Koffler Scientific Reserve, Ontario, Canada [95]; ca. 85% for the Japanese pteridophyte flora [96]; and high (no percentage given) for NW-European ferns [87]. In other geographically restricted barcoding studies, species resolution was considerably lower: up to 70% for tropical tree species in French Guiana [97], and 69.4–74.9% for the flora of Wales [98], for example.
Despite their inability to unambiguously identify all plant species, plant DNA barcodes will be useful in many situations [51]. Already the application of plant DNA barcoding has been demonstrated in a number of studies, including an analysis of spatial variation in root diversity [99], accurate identification of a horticultural fern [100], authenticity of natural health products [101], and diet analyses of herbivorous animals [102] and leaf beetles [103]. Analyses of barcode data have also aided the discovery of new plant and algae species [104,105].
Barcoding Arctic Plants
Barcode data have been produced for some Arctic plant species, but coverage is incomplete. The trnL(UAA) intron and its P6 loop have been used as a DNA barcode for Norwegian Arctic plants [50], and these data have been used to study plant–herbivore interactions [106] and composition of past Arctic plant communities based on ancient DNA [107]. Barcode data for the core plastid loci are available for 23 species of Carex L. and Kobresia Willd. (Cyperaceae) from the Canadian Arctic Archipelago [108], and data for the core plastid regions plus ITS2 were recently published for 312 of 354 vascular plant species known from Churchill, Manitoba, at the southern edge of the Arctic in central Canada [109]. In this study, the three barcode regions combined identified 69% of species, while rbcL + matK identified 54% of species.
Advancement of a DNA barcode library for Arctic plants will facilitate plant identification for taxonomic research, vegetation monitoring, as well as floristic and ecological studies, and will contribute to knowledge of genetic diversity in Arctic plants. As barcode data accumulate for arctic-alpine plant species from throughout their global ranges, the growing barcode library may also contribute to our understanding of the origins and distribution of Arctic flora.
Here we report new DNA barcode data for the core plastid regions (rbcL + matK) for 490 Arctic and northern vascular plant species (over 500 taxa) from Canada, and data for three supplementary plastid loci (atpF–atpH, psbA–trnH, psbK–psbI) for a subset of Puccinellia Parl. and Poa L. species. We characterize the ability of the barcode loci to discriminate genera, species, and infraspecific taxa, and in a subset of genera we explore patterns of genetic variation from taxonomic, phylogenetic, and phylogeographic perspectives.
Methods
Taxon Sampling
The study includes 2644 individuals representing 490 vascular plant species plus 30 additional infraspecific taxa from 50 plant families. Plant material used in the study comes from throughout the Canadian Arctic, with a few specimens from the adjacent boreal region (e.g., Yellowknife, Northwest Territories) and Alaska, U.S.A. (Figure 1). The number of individuals sampled per taxon ranged from 1–27 (mean = 5.0 ± 4.2) (Figure S1). All data for the project were managed in the Barcode of Life Systems (BOLD) database [110] in a project called "Flora of the Canadian Arctic" (project code FCA). Detailed voucher information, including the scientific names of taxa sampled, locality information, collection dates, collectors and collection numbers, herbarium accession numbers, and GenBank accession numbers for all sequences are given in Data Set S1. All voucher specimens were annotated with a label recording their corresponding BOLD Sample ID. Voucher specimens were scanned with Epson flatbed scanners at a resolution of 600 dots per inch, and images were uploaded to BOLD.
Figure 1. Map of sample locations in Canada and Alaska, U.S.A.
A large proportion of the barcode data was generated from silica-gel dried leaf tissue samples collected in the western Arctic as part of floristic studies undertaken by the authors in 2008, 2009, and 2010 (J.M. Saarela and L.J. Gillespie, unpublished data, [29,30]), and field trips throughout the Canadian Arctic Archipelago by Gillespie and L.L. Consaul in the 1990s and 2000s. Fieldwork on Victoria Island, Nunavut, in 2008 was conducted under Nunavut Wildlife Research Permit No. WL 2008-1039 and Nunavut Water Board Licence No. 3BC-AFP0813. Fieldwork on mainland Northwest Territories in 2009 was conducted under Aurora Research Institute Licence No.14524, Inuvialuit Land Administration Licence No. ILA09PN009, and with permission from Parks Canada to conduct fieldwork in Tuktut Nogait National Park of Canada. Fieldwork on Victoria Island, Northwest Territories in 2010 was conducted under Aurora Research Institute Licence No. 14733 and Inuvialuit Land Administration Licence No. ILA10HN004. Voucher specimens for these collections are housed in the National Herbarium of Canada (CAN), Canadian Museum of Nature, Natural Heritage Campus, Gatineau (Data Set S1).
In addition to field-collected material, we also sampled species from CAN herbarium specimens, to expand our taxonomic coverage and geographic sampling. Where possible, we sampled the most recently collected herbarium specimens available, and in most cases we only sampled herbarium material that was green, indicative of fast drying. Sampled herbarium specimens were collected between 1950 and 2010. A small subset of samples was amplified and sequenced from existing DNA extracts.
Taxonomy
Higher-level taxonomy follows recent family-level classifications for lycophytes [111], gymnosperms [112], monilophytes [113], and angiosperms [114]. For nomenclature at the genus, species, and infraspecific levels, we considered available taxonomic information in combination with our experiences with the Arctic flora, as there are many conflicting treatments of species and/or species complexes, even among recent literature [20]. Many Arctic taxa have been variously recognized as species, varieties, or subspecies with little consensus on an appropriate rank, and barcode data may provide new insights that could be taxonomically informative. We thus made a concerted effort to identify taxa to infraspecific rank, even when only one infraspecific taxon was present in our data set, expecting that future growth of the barcode reference library will add infraspecific taxa not sampled here. In most cases infraspecific taxa are represented by the nominal subspecies or variety and one additional infraspecific taxon, and for a few species we sampled more than two additional infraspecific taxa.
We identified our recent field collections and confirmed or re-determined all sampled herbarium specimens through extensive consultation with older [22–24] and recent Arctic floras [25,115], available volumes of the Flora of North America series [116], and the Annotated Checklist of the Panarctic Flora (PAF): Vascular Plants [20]. For many genera, particularly those that are taxonomically difficult, we also consulted the primary taxonomic literature (e.g., Arctagrostis Griseb. [117], Braya Sternb. & Hoppe [118], Chrysosplenium L. [119], Draba L [120]., Eriophorum L. [121], Festuca L. [122–124], Juncus L [125]., Luzula DC [126]., Papaver L. [127], Pedicularis L. [128], Petasites Mill. [129,130], Poa [131–133], Puccinellia [134–143]). All willows (Salix L.) were determined by G.L. Argus (Canadian Museum of Nature). A subset of the Draba, Papaver, Potentilla L., and Hippuris L. material was identified by R. Elven (Natural History Museum, University of Oslo). Nomenclatural information was also obtained from the online databases VASCAN [144,145], Tropicos [146], the International Plant Names Index [147], and the Taxonomic Names Resolution service (http://tnrs.iplantcollaborative.org; version 3.0; accessed December 2012).
DNA Sequencing and Alignment
DNA extraction, amplification, and sequencing of matK and rbcL followed the protocols of the Canadian Centre for DNA Barcoding (CCDB) [148–153], as summarized in Kuzmina et al. [109]. rbcL was amplified and sequenced using the primers rbcLa–F (Levin et al. [154], modified from Soltis et al. [155]) and rbcLa–R (Kress et al. [94], modified from Fofana et al. [156]). matK was amplified in two successive rounds, as necessary. First, all samples were amplified with matK–1RKIM–f and matK–3FKIM–r [157]. Failed samples from the first PCR round were then amplified with matK–390f and matK–1326r [158]. All primer sequences can also be found on the CCDB Protocols website [157]. For a subset of Poa and Puccinellia individuals (Data Set S1) we also sequenced psbA–trnH (20 taxa, 57 individuals; primers [159]:), psbK–psbI (20 taxa, 57 individuals), and atpF–atpH (19 taxa, 56 individuals). Unpublished primers for psbK–psbI and atpF–atpH were designed by K.J. Kim (School of Life Sciences and Biotechnology, Korea University, Seoul, Korea; see Fazekas et al. [34]).
Sequence chromatograms were edited and assembled using CodonCode Aligner 3.7 (CodonCode Co. USA), Sequencher 4.7 (Gene Codes Corporation, Ann Arbor, MI, USA) and Geneious version 6.0.3 (created by Biomatters, available from http://www.geneious.com/). All traces were assembled into contigs and edited manually. Consensus sequences were generated and aligned using MUSCLE [160] as implemented in CodonCode Aligner, Geneious and BOLD. These alignments were examined by eye to detect potential base calling errors, particularly at the beginning and ends of traces. Potential errors were checked in the trace files and corrected as necessary. This process was undertaken multiple times as sequence data were generated and with the final complete data set.
Barcode Validation
Barcode data were validated (quality control) iteratively throughout data collection to identify potential contamination, misidentification, and alignment error. As sequence data were generated, neighbour joining trees (phenograms) using a K2P distance model were generated in BOLD for each plastid region, including all taxa in the data set. In these trees we looked for individuals that were grossly misplaced with respect to their correct families or genera. We also generated trees for single families, in which branch lengths were readily visible compared with the compressed branch lengths in multi-family trees, allowing us to more easily identify potential problems. Individuals that did not cluster with other individuals of their species or species-groups were flagged for follow-up. Voucher specimens of all problematic samples were re-examined, misidentifications were corrected, and clear instances of contamination were removed from the project. In genera with low or no variation among species and/or species-groups, identifying potential identification errors based on the neighbour joining trees was difficult or impossible, as there was little or no informative clustering of taxa to guide this process.
When misidentifications were corrected on specimens, taxon names and the name of the most recent identifier were also updated in BOLD. BOLD does not track identification changes, thus in most cases we included brief comments in the "Taxonomy Notes" field on the BOLD specimen page recording that the determination of the specimen had changed; we also noted the previous and current identifications, the date, and the identifier. This information is the only indication to a BOLD user that a determination of a specimen has changed, as we did not re-image the re-determined voucher specimens.
Genetic Diversity
Infraspecific and interspecific (within-genus) uncorrected p-distances were calculated for all pairwise combinations for rbcL and matK based on unambiguous family-level alignments for each marker using the "Pairwise Summary" function in the program TaxonDNA [161]. The distributions of genetic distances are summarized in a single histogram.
Sequence Recoverability
We calculated the number of rbcL and matK sequences in the entire data set, from samples obtained from herbarium specimens, and from samples obtained from silica-gel dried leaf samples. To determine if herbarium specimen age and sequence recovery are correlated, we divided the herbarium specimens into seven decade-long age classes (and one age class of one year with a single specimen representing the current decade) and counted the number of rbcL and matK sequences recovered from specimens in each age class. We then used a Spearman rank correlation to test for a relationship between these variables. Spearman rank correlation was calculated in PAST [162]. The matK analysis excluded Dryopteridaceae, Equisetaceae, Juncaceae, Polypodiaceae, and Lycopodiaceae, as all samples from these families failed for this marker due to primer mismatch.
Barcode Success
We use the terms 'resolved', 'species resolution', 'discriminated', and 'discrimination success' in reference to taxa with unique DNA barcodes in the current data set. To examine species discrimination for the plastid barcodes, we conducted distance-based and sequence-similarity analyses. Distance-based analyses, which are useful for visualizing patterns of genetic variation, were based on neighbour joining trees generated from uncorrected p-distances [163,164]. Global matrices were initially aligned using BOLD and the Geneious MUSCLE plugin, and examined in Geneious showing codons to ensure the matrices were in frame; these alignments were adjusted by eye as necessary. The global rbcL alignment was straightforward and unambiguous, whereas the matK alignment had several problematic regions, primarily due to insertion/deletion events. To eliminate the effects of possible alignment errors in our analyses, we generated separate rbcL and matK alignments for each family and conducted neighbour joining analyses with these. At the family level, matK was straightforward and unambiguous to align. Single-family neighbour joining trees were much easier to score than multi-family trees. Short branch lengths in the latter, particularly within genera and species complexes, were difficult or impossible to see because of high genetic variation among families.
To determine the discrimination success of a two-locus barcode, the rbcL and matK matrices for each family were concatenated in Geneious into a single matrix. Individuals lacking data for one of the markers were deleted, resulting in a matrix in which all individuals have data for both loci. Neighbour joining trees were generated independently for psbA–trnH, psbK–psbI, and atpF–atpH. Six base pair microinversions were present in psbA–trnH in 14 Puccinellia sequences (BOLD Sample IDs: FCA1084-10, FCA1068-10, FCA1069-10, FCA1070-10, FCA1084-10, FCA1089-10, FCA1090-10, FCA1099-10, FCA1102-10, FCA1105-10, FCA1106-10, FCA1111-10, FCA1118-10, FCA1119-10) and nine Poa sequences (FCA1134-10, FCA1122-10, FCA1123-10, FCA1124-10, FCA1125-10, FCA1126-10, FCA1127-10, FCA1146-10, FCA1148-10) at positions 527 to 532 in the alignment. These inversions were reoriented in the matrix so that all sequences had the same inversion state, and these corrected sequences were uploaded to BOLD.
We scored discrimination in neighbour joining trees by determining the number of genera, species, and infraspecific taxa that could be unambiguously identified in the trees based on their clustering patterns and branch length variation. We did not consider topological monophyly a necessary criterion for taxon discrimination. A genus was scored as discriminated if it shared no identical sequences with other genera. A species was scored as discriminated if its individuals shared no identical sequences with other species. Typically, conspecific individuals clustered together, although in several instances this was not the case. Sometimes, conspecific individuals formed two or more clusters distinct from each other and from all other taxa. Discrimination of infraspecific taxa was scored in the same manner as species when more than one infraspecific taxon was represented in the data sets.
To test species discrimination based on sequence similarity, we conducted BLAST searches following the methods of Burgess et al. [95]. We assembled separate databases of all matK and rbcL sequences. We did not conduct BLAST searches for combined loci, as multilocus searches cannot be conducted in GenBank or BOLD. For each database we conducted all-to-all BLAST searches, querying each sample to the database using the BLASTN plugin in Geneious. For each BLAST search we scored match success at the genus, species, and infraspecific levels. At the genus level, an individual was scored as discriminated when there were no 100% similarity matches with an individual of another genus, regardless of whether the query sequence was more similar to sequences from another genus than to congeneric sequences, as was sometimes the case (e.g., in non-monophyletic genera such as Minuartia). A genus for which all sampled species were correctly assigned was considered discriminated. A species was scored as discriminated if the sequences of all its individuals were distinct from those of all other species. A species was scored as not discriminated if one or more individuals had barcodes identical to those from another species. Species represented by only one individual were scored as discriminated if the individual’s sequence was unique in the database. For species with more than one infraspecific taxon in the database, discrimination success at this level was also scored. An infraspecific taxon was scored as discriminated if all sequences for this taxon differed from those of conspecific infraspecific taxa.
Results
Sequence Recoverability
For the core barcode loci we obtained 4610 new sequences (2150 and 2460 for matK and rbcL, respectively) from 2644 specimens representing 50 plant families, 178 genera, 490 species, 30 additional infraspecific taxa, and 13 unnamed putative hybrids. The number of rbcL and matK sequences recovered per plant family ranged from 1–498 (mean = 49.2 ± 88.0) and 0-486 (mean = 43 ± 84.6), respectively (Figure S2). Both markers were recovered from more than 100 individuals in Poaceae, Cyperaceae, Brassicaceae, Asteraceae, Caryophyllaceae, Saxifragaceae, Salicaceae, and Rosaceae (listed in descending order by number of individuals sampled; Figure S2). Sequence recoverability was significantly higher for rbcL than matK (93% and 81.3% of specimens, respectively; two sample Z-test, Z = 12.7, p = 0; Figure 2). Combined rbcL + matK sequence data were obtained from 75% of the specimens sampled (Figure 2). rbcL sequences were recovered from more families, genera, and species than were matK sequences. We obtained rbcL data for 100% of the 50 families sampled, matK for 86%, and rbcL + matK for 82%; we obtained rbcL data for 98% of the 178 genera sampled, matK for 90%, and rbcL + matK for 86%; we obtained rbcL data for 98% of the 490 species sampled, matK for 88%, and rbcL + matK sequences for 8% (Figure 2). We did not recover any matK sequences in Pinales (Cupressaceae, Pinaceae), monilophytes (Dryopteridaceae, Equisetaceae, Lycopodiaceae, Ophioglossaceae, Polypodiaceae), or one monocot family (Juncaceae) (Figure S2).
Figure 2. Percentage of specimens, families, genera, species, additional infraspecific taxa, and unnamed hybrids in the data set from which rbcL and matK barcodes were recovered.
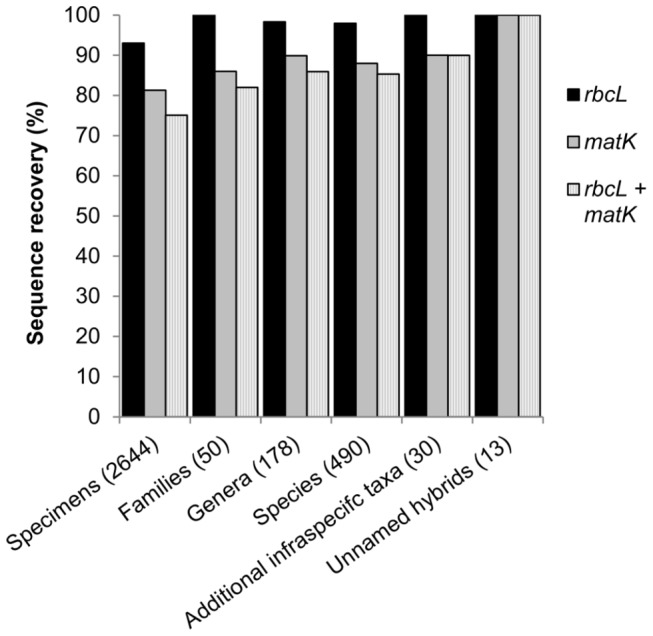
Numbers in parentheses are the total number of individuals (specimens, unnamed hybrids) and taxa (families, genera, species, additional infraspecific taxa) in each category in the data set.
There was no difference in rbcL recovery from herbarium specimens or silica-gel dried material (92.3% for herbarium specimens and 93.1% for silica-gel dried samples; two sample Z-test, Z = -0.841, p = 0.20045). In contrast, matK recovery differed significantly for source materials (76.5% for herbarium specimens and 85.1% for silica-gel dried samples; two sample Z-test, Z = -5.11, p = 0). Excluding monilophyte, lycophyte, and conifer taxa (all of which failed for matK regardless of sample source due to primer mismatch), matK was recovered from 80.2% of herbarium specimens sampled and 84.9% of silica-gel dried samples (two sample Z-test, Z = -5.678, p = 0). Age of herbarium specimen and recovery of matK were significantly correlated (Spearman's rank correlation, p < 0.05, Figure 3), whereas there was no relationship between these variables for rbcL (Spearman's rank correlation, p > 0.05, Figure 3).
Figure 3. Relationship between herbarium specimen age and sequence recovery (%) for rbcL and matK.
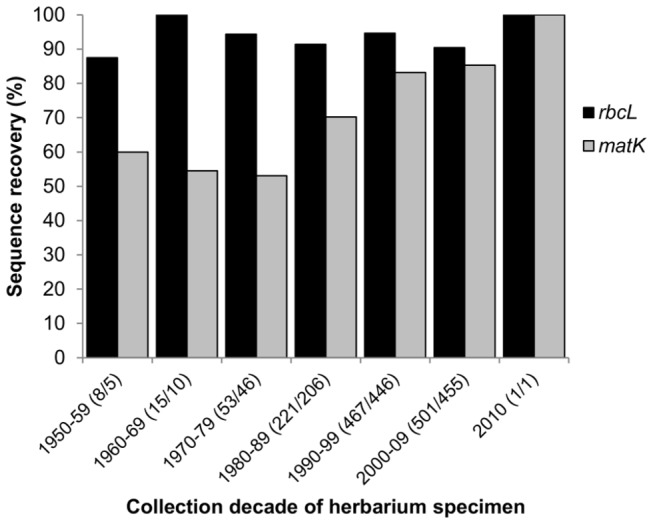
Material was sampled from 1169 herbarium specimens collected between 1950–2010. These were divided into seven age classes and a Spearman rank correlation was used to test for a relationship between age class and percent recovery of rbcL (Spearman's rho 0.306, p = 0.50079) and matK (Spearman's rho 0.893, p = 0.012302). The matK analysis excluded Dryopteridaceae, Equisetaceae, Juncaceae, Polypodiaceae, and Lycopodiaceae, which failed for all samples for this marker due to primer mismatch. Numbers in parentheses are the total number of herbarium specimens sampled from each age class for rbcL and matK, respectively.
Genetic Diversity
Based on genetic divergence within and among congeneric species, matK is ca. three times more divergent than rbcL at the infraspecific level (mean pairwise divergence: matK = 0.000905 ± 0.002226, rbcL = 0.000299 ± 0.001327) and ca. 2.6 times more divergent than rbcL at the interspecific level (mean pairwise divergence: matK = 0.010686 0.01579, rbcL = 0.00404 ± 0.006439). A few matK interspecific pairwise comparisons differ by more than 10% (Figure 4). Frequency distribution of intra- and interspecific divergences showed that ranges of genetic distances within species and among congeneric species are considerably greater for matK than for rbcL (Figure 4). For both markers there was considerable overlap between infraspecific and interspecific genetic distances.
Figure 4. Frequency distribution of infraspecific and congeneric interspecific genetic divergences of rbcL and matK.

Numbers in parentheses are the total number of comparisons for each category. Divergences were calculated using uncorrected p-distances.
Barcode Success
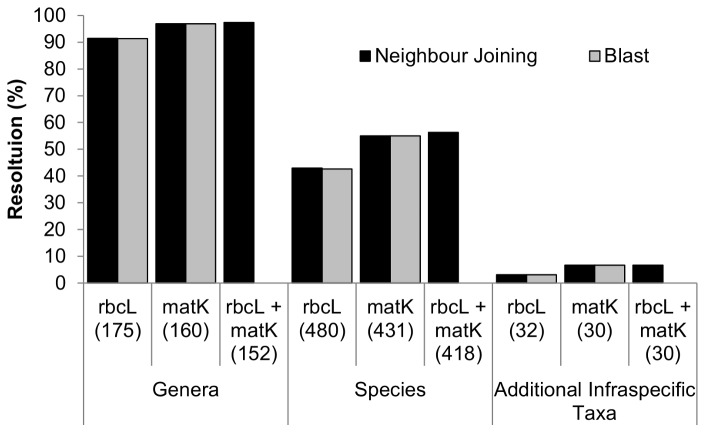
We determined percent species resolution based on BLAST searches and neighbour joining trees generated from uncorrected p-distances calculated from single-family alignments. Neighbour joining trees for rbcL, matK, and rbcL + matK for each family are presented in Figures S3–S47; trees are not presented for Araceae, Ophioglossaceae, and Santalaceae, as only one or two individuals in these families were sampled. For both BLAST and neighbour joining analyses, a species was considered resolved if all members had barcodes that differed from all individuals of other species. Levels of discrimination were identical or nearly identical for BLAST searches and neighbour joining trees. In both BLAST and neighbour joining analyses, over 90% of sampled species could be assigned to their genus by rbcL (91.4%), matK (96.9%), or rbcL + matK (97.4%) (Figure 5). Discrimination of species was 42.6–42.9% based on rbcL, 55% based on matK, and 56.3% based on rbcL + matK (Figure 5). Discrimination of infraspecific taxa was low for all markers, ranging from 3.1% for rbcL to 6.7% for matK and rbcL + matK (Figure 5).
Figure 5. Resolution (%) of genera, species, and additional infraspecific taxa for rbcL, matK, and rbcL + matK in neighbour joining trees and BLAST searches.
Neighbour joining trees were generated from single-family alignments using uncorrected p-distances. Numbers in parentheses are the numbers of genera or species sampled in each data set. BLAST searches were not conducted for the combined rbcL + matK data.
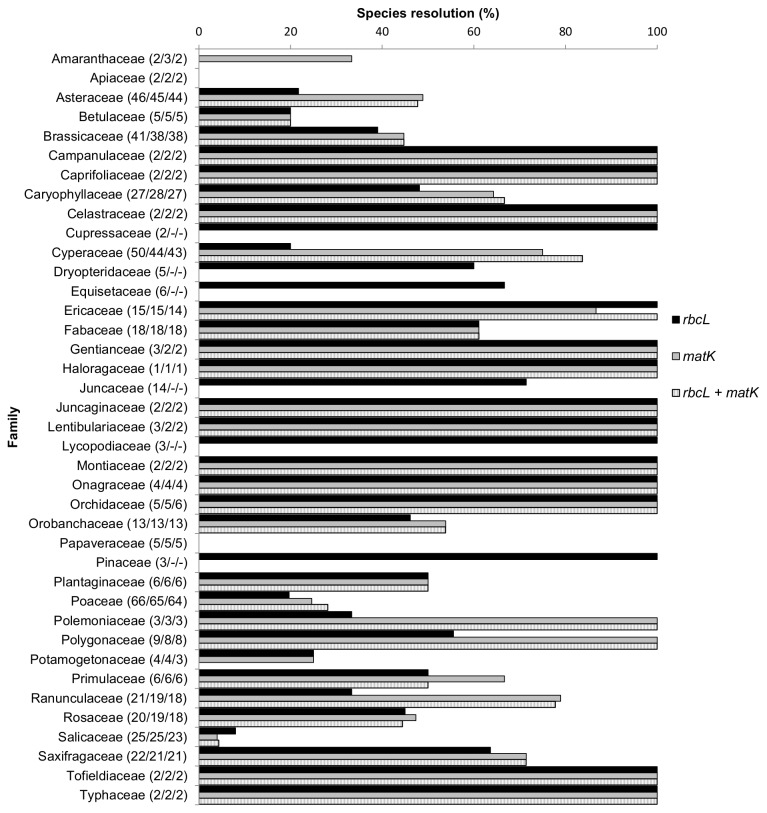
At the family level, species resolution ranged from 0% to 100% (Figure 6). Resolution of species with rbcL + matK in nine families from which 20 or more species were sampled (Poaceae, Cyperaceae, Asteraceae, Brassicaceae, Caryophyllaceae, Salicaceae, Saxifragaceae, Rosaceae, Ranunculaceae; listed in descending order by number of species sampled) ranged from 4% (Salicaceae) to 84% (Cyperaceae) (Figure 6). In 29 genera the rbcL + matK barcode provided 100% species resolution; in these genera two to four species were sampled (Table 1). By contrast, in 21 genera with two or more species sampled for the two-locus barcode there was 0% species resolution (Table 2). In four genera, one species per genus was sampled and none were resolved (Table 2); these taxa are not distinct at the genus level. Species resolution in the ten genera with ten or more sampled species (Carex, Draba, Festuca, Pedicularis, Poa, Potentilla, Puccinellia, Ranunculus L., Salix, Saxifraga L.) ranged from 0% to 94%; in seven of these less than 50% of species were resolved (Figure 7). For both individual and combined data sets, there was a significant negative correlation between the number of species sampled per genus and the percent species resolution per genus (Figure 8).
Figure 6. Species resolution (%) per family for rbcL, matK, and rbcL + matK.
Numbers in parentheses refer to the numbers of species for which barcode data were recovered for rbcL, matK, and rbcL + matK, respectively. Families with a single genus and species sampled are excluded. Dashes (-) indicate that no sequences were recovered.
Table 1. Genera in which all species are resolved by rbcL, matK, and/or rbcL + matK.
| Family | Genus |
Number of species sampled/resolved
|
||
|---|---|---|---|---|
| rbcL | matK | rbcL + matK | ||
| Asteraceae | Arnica Rupp. ex L. | 2/0 | 2/2 | 2/2 |
| Artemisia L. | 3/1 | 3/3 | 3/3 | |
| Brassicaceae | Arabidopsis (DC.) Heynh. | 2/2 | 2/2 | 2/2 |
| Cardamine L. | 4/4 | 4/4 | 4/4 | |
| Parrya R. Br. | 2/2 | 2/2 | 2/2 | |
| Campanulaceae | Campanula L. | 2/2 | 2/2 | 2/2 |
| Caryophyllaceae | Arenaria L. | 2/0 | 2/2 | 2/2 |
| Sagina L. | 2/2 | 2/2 | 2/2 | |
| Stellaria L. | 3/1 | 3/3 | 3/3 | |
| Cyperaceae | Parnassia L. | 2/2 | 2/2 | 2/2 |
| Kobresia Willd. | 3/2 | 3/3 | 3/3 | |
| Ericaceae | Arctous (A. Gray) Nied. | 2/2 | 2/0 | – |
| Rhododendron L. | 2/2 | 2/2 | 2/2 | |
| Vaccinium L. | 2/2 | 2/2 | 2/2 | |
| Fabaceae | Hedysarum L. | 2/2 | 2/2 | 2/2 |
| Juncaginaceae | Triglochin L. | 2/2 | 2/2 | 2/2 |
| Utriculariaceae | Pinguicula L. | 2/2 | 2/2 | 2/2 |
| Onagraceae | Chamerion (Raf.) Raf. ex Holub | 2/2 | 2/2 | 2/2 |
| Epilobium L. | 2/2 | 2/2 | 2/2 | |
| Orchidaceae | Platanthera Rich. | 3/1 | 3/3 | 3/3 |
| Plantago L. | 2/2 | 2/2 | 2/2 | |
| Polemoniaceae | Phlox L. | 2/0 | 2/2 | 2/2 |
| Polygonaceae | Bistorta (L.) Scop. | 2/2 | 2/2 | 2/2 |
| Rumex L. | 4/0 | 4/4 | 4/4 | |
| Primulaceae | Androsace L. | 2/2 | 2/2 | 2/2 |
| Ranunculaceae | Anemone L. | 3/3 | 3/3 | 3/3 |
| Rosaceae | Rubus L. | 2/2 | 2/2 | 2/2 |
| Salicaceae | Populus L. | 2/2 | – | – |
| Saxifragaceae | Chrysosplenium L. | 3/3 | 3/1 | 3/3 |
| Tofieldiaceae | Tofieldia Huds. | 2/2 | 2/2 | 2/2 |
| Typhaceae | Sparganium L. | 2/2 | 2/2 | 2/2 |
A dash (-) indicates that only one species was sampled for a locus or the combined loci.
Table 2. Genera in which no sampled species are resolved by rbcL, matK, and/or rbcL + matK.
| Family | Genus |
Number of species sampled/resolved
|
||
|---|---|---|---|---|
| rbcL | matK | rbcL + matK | ||
| Amaranthaceae | Suaeda Forssk. ex J.F. Gmel. | 2/0 | 2/0 | 2/0 |
| Apiaceae | Bupleurum L. | 2/0 | 2/0 | 2/0 |
| Asteraceae | Antennaria Gaertn. | 6/0 | 4/0 | 4/0 |
| Arctanthemum (Tzvelev) Tzvelev | 1/0 | 1/1 | 1/1 | |
| Arnica Rupp. ex L. | 2/0 | 2/2 | 2/2 | |
| Eurybia (Cass.) Gray | 1/0 | 1/1 | 1/1 | |
| Hulteniella Tzvelev | 1/0 | 1/1 | 1/1 | |
| Petasites Mill. | 2/2 | 2/0 | 2/0 | |
| Saussurea DC. | 2/0 | 2/0 | 2/0 | |
| Solidago L. | 2/0 | 2/0 | 2/0 | |
| Symphyotrichum Nees | 1/0 | 1/1 | 1/1 | |
| Taraxacum F.H. Wigg. | 8/0 | 8/1 | 8/1 | |
| Tephroseris (Rchb.) Rchb. | 3/0 | 3/0 | 3/0 | |
| Betulaceae | Betula L. | 4/0 | 4/0 | 4/0 |
| Brassicaceae | Braya Sternb. & Hoppe | 4/0 | 4/1 | 4/1 |
| Draba L. | 18/1 | 16/0 | 16/0 | |
| Erysimum L. | 3/0 | 2/0 | 2/0 | |
| Transberingia Al-Shehbaz & O'Kane | 1/0 | 1/1 | 1/1 | |
| Caryophyllaceae | Arenaria L. | 2/0 | 2/2 | 2/2 |
| Cerastium L. | 4/0 | 4/0 | 4/0 | |
| Cupressaceae | Juniperus L. | 2/0 | 2/2 | 2/2 |
| Cyperaceae | Eriophorum L. | 7/0 | 7/0 | 7/1 |
| Papaveraceae | Papaver L. | 5/0 | 5/0 | 5/0 |
| Plantaginaceae | Hippuris L. | 3/0 | 3/0 | 3/0 |
| Poaceae | Agrostis L. | 2/0 | 2/0 | 2/0 |
| Anthoxanthum L. | 3/0 | 4/1 | 4/1 | |
| Arctagrostis Griseb. | 1/1 | 1/0 | 1/1 | |
| Arctophila (Rupr.) Andersson | 1/0 | 1/0 | 1/0 | |
| Calamagrostis Adans. | 5/0 | 5/0 | 5/0 | |
| Deschampsia P. Beauv. | 3/0 | 3/0 | 3/0 | |
| Dupontia R. Br. | 1/0 | 1/0 | 1/0 | |
| Elymus L. | 4/0 | 4/0 | 4/0 | |
| Koeleria Pers. | 1/0 | 1/0 | 1/0 | |
| Phippsia (Trin.) R. Br. | 2/0 | 2/0 | 2/0 | |
| Trisetum Pers. | 1/0 | 1/0 | 1/0 | |
| Polemoniaceae | Phlox L. | 2/0 | 2/2 | 2/2 |
| Polygonaceae | Rumex L. | 4/0 | 4/4 | 4/4 |
| Potamogetonaceae | Stuckenia Börner | 3/0 | 3/1 | 3/0 |
| Primulaceae | Primula L. | 3/0 | 3/0 | 3/0 |
| Ranunculaceae | Coptidium (Nyman) Tzvelev | 2/0 | 1/1 | – |
| Ranunculus L. | 12/0 | 11/7 | 11/7 | |
| Salicaceae | Salix L. | 22/0 | 24/0 | 22/0 |
A dash (-) indicates no species were sampled for the combined loci.
Figure 7. Species resolution (%) in ten genera with the greatest number of species sampled.
Numbers in parentheses refer to the number of species sampled for rbcL, matK, and rbcL + matK, respectively.
Figure 8. Scatterplots of the number of species sampled in each genus against the percentage of species resolved in each genus with rbcL, matK, and rbcL + matK.

A. rbcL-175 genera (Pearson correlation coefficient r = 0.4180, n = 175, P < 0.0001), R2 = 0.1747. B. matK-159 genera (Pearson correlation coefficient r = 0.3685, n = 159, P < 0.0001), R2 = 0.1358. C. rbcL + matK-153 genera (Pearson correlation coefficient r = 0.3636, n = 153, P < 0.0001), R2 = 0.1322. Species resolution was scored in neighbour joining trees generated from uncorrected p-distances calculated from single-family alignments.
Thirty species from 11 families include more than one infraspecific taxon (51 infraspecific taxa in total; Table S1, Data Set S1). Five infraspecific taxa were distinguished with the barcode data: Oxytropis borealis DC. var. Borealis, O. Borealis var. viscida (Nutt.) S.L. Welsh, Eriophorum scheuchzeri Hoppe subsp. scheuchzeri, E. scheuchzeri subsp. arcticum M.S. Novos., and Stuckenia filiformis (Pers.) Börner subsp. filiformis (Table S1).
The supplementary plastid loci psbA–trnH, atpF–atpH, and psbK–psbI were sequenced for a subset of Poa species and additional infraspecific taxa and Puccinellia species (Data Set S1, Figures S49, S50, S51). psbA–trnH discriminated 50% of Poa species, 38% of Poa taxa (i.e., species and additional infraspecific taxa), and no Puccinellia species; psbK–psbI discriminated 17% of Poa species, 10% of Poa taxa, and no Puccinellia species; and atpF–atpH discriminated 33% of Poa species, 20% of Poa taxa, and 44% of Puccinellia species (Table S3).
Discussion
Sequence Recoverability
A key criterion for a standard land plant barcode is universality, meaning that the DNA barcode should be easily recovered from all land plants, ideally with a single primer pair [52]. Our amplification and sequencing success was greater for rbcL than matK, consistent with the results of numerous other studies that sampled broadly across land plants (e.g., [52,58,94,95,109]). Recovery of rbcL was high (93.1%), similar to the results of other studies focused primarily on angiosperms in which rbcL recovery ranged from 90–100% [34,52,58,79,95,109,165]. The single pair of rbcL primers we used worked in gymnosperms, lycophytes, monilophytes, and angiosperms, as other studies have also found (e.g., [34]). Although other primers have been used to recover rbcL from ferns [87,96] and gymnosperms in DNA barcoding studies (e.g., [166]), a single set of primers that will amplify rbcL across land plants is of great practical use, facilitating recovery of the region from any unknown sample. In angiosperms, rbcL was recovered from an equal or greater number of samples per family than matK, except in Boraginaceae, in which rbcL was recovered from just one of 11 samples, which all worked for matK (Figure S2). The reasons for this high rbcL failure in Boraginaceae are unclear.
In contrast to rbcL, recoverability of matK differed substantially among the major land plant lineages. No matK sequences were recovered from gymnosperm, lycophyte, and monilophyte taxa (Figure S2). These results are similar to previous studies, which have found that matK primers used in angiosperms generally work poorly in gymnosperms [45,52,98,167–169]. matK has been successfully recovered in gymnosperms using taxon-specific primers [34,61], and a new set of matK primers with high PCR universality, high sequence quality, and high coverage across gymnosperms has been recently recommended for barcoding gymnosperms [167].
Previous studies have similarly not been successful in recovering matK from ferns with the universal primers widely used in DNA barcoding studies [37,96,109]. These matK primers do not work because of the loss of trnK and its intron in most leptosporangiate ferns, including the trnK exons in which the priming sites for the angiosperm-designed primers are located [170-172]. Among the fern taxa sampled here, Botrychium Sw. (Ophioglossaceae) and Equisetum L. (Equisetaceae) are non-leptosporangiate genera [113] and have trnK genes, yet the universal primers still did not amplify matK for these taxa, likely reflecting variation in the priming sites. Acknowledging the difficulty of amplifying matK in ferns, DNA barcode data have been generated for regional fern floras using combinations of rbcL and trnH–psbA [96] and rbcL and trnL–F [87], excluding matK from consideration. Nevertheless, matK has recently been demonstrated to be variable and useful as a DNA barcode for ferns in studies using primers specific to particular genera or lineages [172,173]. These should be tested for Arctic ferns.
Among angiosperms we recovered matK sequences from all families except Juncaceae (Figure S2). Results of other studies confirm that sequencing matK is problematic in this family with the primers we used. For example, matK failed for nearly all Juncaceae taxa sampled from Churchill, being successful in just two of 33 samples [109], and Burgess et al. [95] obtained sequence data for only two of five sampled Juncaceae taxa from southern Canada. Alternate matK primers have provided better results: de Vere et al. [98] amplified and sequenced some 40 of 76 sampled Juncaceae taxa using primers they designed for Poales, though still had a nearly 50% failure rate, whereas Schaefer et al. [174] sequenced nine sampled Juncus taxa using matK primers they newly designed. These latter two sets of primers should be tested across the family.
Recovery of matK ranged considerably in the other angiosperm families. Considering only specimens sequenced from silica-gel dried leaf material (from which sequence recovery is not expected to be affected by sample age) and 14 families with more than 15 individuals sampled (not including Juncaceae), matK failure was less than 10% in ten families, but considerably higher in the remainder (Table S2). The greatest failure to recover matK occurred in Saxifragaceae (46.8% failure), Ranunculaceae (26.1%), Polygonaceae (22.7%), and Cyperaceae (22.6%) (Table S2). In all of these families there was no clear pattern to the failures: in most cases, some individuals of a species failed while other individuals of the same species were successful; an exception to this is Saxifraga oppositifolia L., from which we recovered matK from just one of ten samples. These results are generally similar to those of de Vere et al. [98], who found recovery of matK from fresh material to be low to moderate in Saxifragales, Ranunculales, and Poales, the orders in which Saxifragaceae, Ranunculaceae, and Cyperaceae are classified. Increased matK failure in these groups may be a primer design issue. In contrast to our inability to recover matK barcodes from 1/5 of our Cyperaceae samples, two studies that generated large amounts of barcode data for Cyperaceae did not report any major problems recovering matK [108,175]. These studies used different primers for this gene (matK 2.1af, matK–2.1f, matK–5r; Royal Botanic Gardens, Kew, www.kew.org/barcoding), which may be better than the primers we used to recover matK in Cyperaceae, particularly in Carex and Kobresia. Given the variability in matK across land plants—the property that makes it useful as a DNA barcode—it is now recognized that a single universal primer pair that will amplify matK in all taxa is likely not realistic [37,176]. Accordingly, new order-specific primers have been designed for matK across angiosperms that increase its recovery significantly [176]. matK primers that have been used in phylogenetic studies are available for Saxifragaceae, as well (e.g., [177–179]).
Over half of the barcodes in our study were recovered from silica-gel dried material collected fresh in the field, and recovery of rbcL and matK sequences from this material was generally quite high (93.1% and 85.1%, respectively). Such recently-collected material, when available, is desirable to work with as it generally performs well in the laboratory, and does not require destructive sampling of existing herbarium collections. Unfortunately, obtaining fresh plant collections from most Arctic areas requires substantial logistical planning and financial resources. Sampling existing herbarium material allowed us to nearly double the number of specimens sampled in our study, expanding considerably our sampling both taxonomically and geographically. Forty-four percent of the DNA extracts we used were obtained from herbarium specimens, collected from 1950 to the present. We found no differences in recoverability of rbcL when sampled from silica-gel dried (93.1%) or herbarium material (92.7%), and the ages of the herbarium specimens we sampled did not affect rbcL recoverability, as in a related study of Arctic plants [109]. This result is not unexpected, as our selection of herbarium material was not random. We chose herbarium specimens collected recently and with material that appeared to have dried rapidly, which likely contributed to the high success of rbcL recovery. By contrast, recoverability of rbcL from herbarium specimens documenting the Welsh flora was 14% lower than from fresh material [98]. Recovery of rbcL from herbarium material in that study may have been lower than observed here because they included material up to nearly a century older (as old as 1868) than did we (as old as 1950).
In contrast to our results for rbcL, we found recovery of matK to be significantly lower (76.5%) from herbarium specimens than from silica-gel dried material (85.1%), and the age of herbarium specimens did have a significant effect on matK recoverability. As the age of the herbarium specimens increased, we failed to recover matK from an increasingly greater proportion of the sampled specimens (Figure 3), as in de Vere et al. [98]. Over 85% of the specimens collected in the last two decades yielded matK sequences, similar to the barcoding study of the Welsh flora, in which matK was recovered from 70% and 80% of specimens collected in each of the last two decades, respectively. Lower recovery of matK from herbarium specimens has been attributed to the longer length of this region (ca. 800 bp), making it more difficult to amplify from degraded DNA, compared to rbcL (552 bp) [109]. Differences in sequence recovery from herbarium specimens among studies is not unexpected, and may reflect differences in local storage conditions of herbarium specimens sampled, the rate of initial drying of the specimens in the field, or the primers used to amplify the gene regions.
Despite an increased number of amplification and sequencing problems with herbarium specimens, they are a critically important source of material for barcoding plants. In general, efforts to generate DNA barcodes from herbarium material should focus on the most recently collected specimens available to maximize successful barcode recovery [98], as our results demonstrate for matK. Identifications of herbarium material being used to generate barcode data should always be confirmed. We found nomenclature on many herbarium specimens that required updating to reflect current taxonomy, and occasionally we encountered herbarium specimens that had more than one species mounted on a sheet (i.e., mixed collections), particularly in genera with morphologically similar species such as Carex and Draba. Mixed collections often became evident only upon re-examining the herbarium sheet of a putatively misplaced individual in a neighbour joining tree, and realizing that the sequence of the suspicious individual came from a second species on the sheet—the one from which leaf material was obtained—which had not been noticed previously. We now routinely mark the individual on a herbarium sheet from which leaf material is removed with a small arrow so it is clear from which plant the barcode data were recovered.
Genus and Species Resolution
The primary goal of DNA barcoding is to assign unknown individuals to known species by matching their sequences with those of known species in a reference library. Multiple approaches have been explored for evaluating the success of plant barcoding markers for identifying species, including phenetic analyses based on distance measures, phylogenetic methods using parsimony, maximum likelihood and Bayesian criteria, comparisons of inter- and infraspecific genetic distances, sequence similarity analyses using Basic Local Alignment Search Tool (BLAST) searches or other algorithms, and character-based approaches (e.g., [161,180–183]). Unfortunately there is no consensus in the plant barcoding literature, or the barcoding literature more broadly, on criteria for unambiguously discriminating taxa with barcode data. At present, the plant identification tool in BOLD uses the BLAST algorithm and accepts rbcL and matK sequences, although it does not accept both simultaneously.
We considered a species to be successfully discriminated when all individuals of a species had barcode sequences not shared by any other species in the data set; we did not apply a bootstrap threshold, we did not require an arbitrary minimum level of genetic variation among species, and we did not require all individuals of a species to cluster together, as we were not testing species monophyly. The latter is problematic with the phenetic approaches used here and reliance on plastid data alone, which represents a single linkage group and does not necessarily reflect organismal phylogeny. Fazekas et al. [34] provide a critical discussion of monophyly as a criterion for determining barcoding success.
Because we have allowed the smallest possible sequence differences (one nucleotide) to discriminate species, we consider the level of resolution obtained here to be at or near the upper limit for the Canadian Arctic flora for the core plant barcoding markers. Application of bootstrap support or genetic variation thresholds would likely result in considerably decreased resolution, given the few nucleotide differences that distinguish many species. A practical limitation of our approach is that an unknown individual with a barcode haplotype not represented in the database for its species may not be assignable to its species if it does not cluster with other conspecific individuals in a neighbour joining tree, or if it does not find a 100% match in a BLAST search. This is an unfortunate reflection of low variation in the core plastid barcode loci among closely related plant species. Extensive sampling from throughout species' geographic ranges will be needed to maximize the probability that all barcode haplotypes for a species are sampled and represented in the barcode reference library. We certainly have not detected the full range of variation in the core barcode loci in most of the nearly 500 species sampled here, considering we sampled only six or fewer individuals per species in over half the species in the data set, and just one individual in 120 of the species studied (Figure S1). Further, we have not sampled any individuals from beyond the northern North American portions of most of the sampled species' ranges, which in most cases are considerably broader, variously extending into southern Canada and the U.S.A., other global Arctic regions to the east and west, and even into the southern hemisphere [20].
Genus Resolution
Multiple studies have demonstrated that the core barcode loci routinely provide high discrimination at the genus level, usually greater than 90% (e.g., [94,98]). Accordingly, we find that rbcL distinguishes 91.4% of genera, and matK and rbcL + matK distinguish 97% of genera in our data set. Considering matK and the combined loci, just four genera—all members of Poaceae—cannot be distinguished. Two of these, Dupontia R.Br. (a genus of one polymorphic species [184,185]) and Arctophila Rupr. (monotypic), are distinct from all other genera, but share identical rbcL and matK barcodes. Dupontia and Arctophila are closely related and form a strongly supported clade in phylogenetic analyses; they are distinguished from each other weakly, based on plastid trnT–F data [186,187], or not at all, based on trnL [184] and ITS data [184,186]. Dupontia may have an intergeneric hybrid origin, with one parent being Arctophila fulva (Trin.) Andersson and the other parent unknown [184]. The other two unresolved genera are also represented by one species each (Koeleria macrantha (Ledeb.) Schult. and Trisetum spicatum (L.) K. Richt.), which have identical rbcL and matK barcodes. These results are consistent with the findings of previous phylogenetic work, in which species of Koeleria Pers., Trisetum Pers. (including T. spicatum), and other (non-Arctic) genera were intermixed—a group of taxa in which generic boundaries are not clear [188,189].
Species Resolution
The core plastid DNA barcode markers, when combined, discriminate a maximum of 56% of the species sampled here. This is a considerable improvement over resolution with rbcL alone (42.6–42.9%) and a slight improvement over matK alone (55% resolution). Although in a few cases rbcL discriminates species that have identical matK barcodes, discrimination with the combined loci is not considerably greater than with matK alone because the discrimination method we used is based on minimum differences between species. Adding distinguishing characters from a second locus does not increase the resolution if the species is already discriminated by the first locus. However, the increased variation in the two-locus barcode is taken into account in other methods for characterizing species discrimination (listed above).
Since the recommendation of rbcL and matK as core DNA barcoding loci in 2009 [52], several large plant barcoding studies have included these markers (Table 3), allowing comparisons of the performance of these loci across a diversity of taxa and floristic regions. A caveat to such comparisons is that different studies often use different criteria for species discrimination. Species discrimination for the Canadian Arctic flora with rbcL + matK is nearly identical to the 54% of 286 species discriminated with these markers for the flora of Churchill, Manitoba [109]. This is not unexpected, as Churchill is located along the southern boundary of the Canadian Arctic ecozone and many of the same species are sampled in both studies. However, given the smaller number of species in the Churchill study, it may be expected that discrimination would be greater in that local flora compared with the broader Arctic flora considered here. Kuzmina et al. [109] used a criterion of monophyly to score species discrimination, a more conservative method than the one we used. Species resolution in the Churchill data set may be greater if the discrimination methods used here were applied. Our results are also similar to those of a larger study in which rbcL + matK discriminated 49.7% of 765 species sampled mostly from China [58].
Table 3. Species resolution (%) with rbcL + matK among floristic studies that sampled multiple families, genera, and species at country and local scales.
| Number of genera/ species in study (number of species analysed for rbcL + matK) | Geographical Scale: Sampling Region | Species resolution with rbcL + matK | Reference |
|---|---|---|---|
| 455/1143 (808) | Country: Wales | 69.4–74.9% | [98] |
| 269/436 (282) | Local: Koffler Scientific Reserve, Ontario, Canada | 93.1% | [95] |
| 147/354 (not given) | Local: Churchill, Manitoba | 54% | [109] |
| 141/1757 (765) | Country: China | 49.7% | [58] |
| 181/296 (205) | Local: tropical forest plots, Panama | 92% | [94] |
A range is given when percent resolution differed among scoring methods.
Species resolution in the Canadian Arctic with rbcL + matK is considerably lower than discrimination rates of 70% or greater reported in other floristic studies for the same two-locus barcode (Table 3). rbcL + matK have provided the highest species resolution in floristic studies that considered only species found in highly restricted areas—a 5 km2 forest plot [94] and a 3.5 km2 field station [95]—limiting the total number of species overall and, more critically, the number of closely related species in the data set. Similarly, discrimination with rbcL + matK in the Welsh flora increased as spatial scales of decreasing size were considered, from up to 74.9% at the country level to 81.6% and 93.3% considering 10 km2 and 2 km2 plots, respectively [98]. These geographically restricted floristic barcoding studies demonstrate the power and utility of the core plant DNA barcoding loci for species discrimination in well-defined local regions, based on reference barcode libraries developed explicitly to support research in those regions. The utility of a local barcode reference library for addressing local ecological questions was demonstrated by Kesanakurti et al. [99], who used a DNA barcode library generated for the flora of a field station to identify roots and characterize below-ground plant diversity at sites in the field station. However, there is not always increased species resolution at smaller geographical scales, as the Churchill study may demonstrate. The barcoding studies of the Welsh flora and Chinese plants each considered a similar number of species and used a similar metric to score species discrimination, yet resolution was considerably higher for the flora of Wales for rbcL + matK (74%) compared with the resolution for Chinese plants with these same markers (49.7%). This likely reflects differences in the number of species sampled per genus, which was considerably higher in the study of Chinese plants [98].
As we sampled fairly extensively across many families, our results can be compared with taxon-specific barcoding studies of particular families and genera. Pang et al. [190] demonstrated very high species discrimination (93–96%) with rbcL and matK among ca. 70 species in Rosaceae from ca. 22 genera, but no more than one or a few species in most genera were included. Our Rosaceae sampling for rbcL + matK includes six genera and 18 species (Figure S43), of which just 44% are resolved. In four of these six genera (Comarum L., Dasiphora Raf., Dryas L., Sibbaldia L.) we sampled a single species and in Rubus L. we sampled two distantly related species [191]. These six species are all distinguished by the barcode data, whereas the remainder of our sampling (12 species) was in Potentilla, in which resolution is poor (see discussion of Potentilla below). A study evaluating barcode markers in Asteraceae similarly found high resolution with rbcL (87.1%) and matK (94.3%) among 63 species in 48 genera from China [192]. The high resolution in this study can be attributed to the one or a few species per genus that were sampled. We sampled 21 genera and 44 species in Asteraceae (Figure S5), and find just 48% species resolution with rbcL + matK. Twelve of these genera include a single species and are distinct at the genus level (and therefore at the species level, as well) whereas the remaining genera include two or more species, most of which are not distinguished by the barcode data. An exception is Artemisia L., in which the three sampled species can be identified. This is not surprising as each of these species is part of a different major clade in the genus [193]. Similarly, the two sampled species of Arnica L. are distinguished by matK; these two species are not closely related [194].
The ability of plastid loci to discriminate species is related to the rate of molecular evolution in a genus or lineage, the length of time that species have been separated, phylogenetic relationships among sampled species and genes, and other evolutionary and biological phenomena such as polyploidy, recent and past hybridization, apomixis, coalescence failure, and introgression (e.g., [34,51]). In general, closely related species are less likely to be distinguished by plastid DNA barcodes compared with those that are more distantly related, as demonstrated in studies that have explicitly considered closely related taxa (e.g., [175,195]). A recent study of the origins and diversification of the global Arctic flora, based on analyses of molecular phylogenetic studies that included Arctic taxa, found that congeneric Arctic species originated mostly independently in unrelated lineages, and that there were few species' radiations in the Arctic [196]. This study considered about 40% of Arctic genera and some 30% of Arctic species. Based on this study, species identification in the Arctic flora and the proportion of species in a genus that can be discriminated should not be greatly affected as the number of species sampled per genus increases, because most species are not expected to be closely related. Contrary to these expectations, however, overall discrimination in the Arctic flora with the core plastid barcodes is low, and, as the number of species sampled per genus increases in the current data set, species resolution tends to decrease (Figure 8).
Resolution of Infraspecific Taxa with DNA Barcodes
Few plant barcoding studies have explicitly considered the ability of barcoding markers to discriminate infraspecific taxa (varieties and subspecies), yet accurate identification of infraspecific taxa can be as important as identification to species level in many avenues of research (e.g., conservation biology, rare species assessments, floristic inventories, phylogeography, etc.). The global Arctic flora contains a large number of infraspecific taxa, many of which have been variously recognized at specific or infraspecific ranks [20]. Although sometimes difficult to identify, most are defined by combinations of morphological characteristics, unique distributions and ploidy differences, all reflecting putatively unique evolutionary origins. Some 30 species from 11 families in our study include more than one infraspecific taxon. Of these all but five cannot be distinguished from one another by the core plastid barcodes (Table S1). This is not surprising given that many closely related species cannot be distinguished by the core barcode loci. There is no infraspecific variation between P. hartzii subsp. hartzii and subsp. vrangelica, the latter a viviparous taxon of plants. This trait is variable within Canadian Arctic populations, and subspecies recognition may not be appropriate in the North American Arctic (LJ Gillespie, personal observation).
In a few species there is infraspecific variation in the barcoding loci that corresponds to infraspecific taxa. rbcL distinguishes Stuckenia filiformis subsp. filiformis from three additional infraspecific taxa: subsp. alpina (Blytt) R.R. Haynes, Les & M. Král, subsp. occidentalis (J.W. Robbins) R.R. Haynes, Les & M. Král and subsp. borealis (Raf.) Tzvelev & Elven (=var. borealis (Raf.) H. St. John). rbcL and matK sequences of S. filiformis subsp. occidentalis are identical to those of S. vaginata and S. subretusa (Hagstr.) Holub. Elven et al. [20] suggested that this taxon may be closer to S. vaginata and S. pectinata (L.) Börner; however, a matK sequence for S. pectinata is more similar to individuals of S. filiformis subspp. alpina and borealis. matK and rbcL + matK distinguish Oxytropis borealis var. borealis from O. borealis var. viscida, and rbcL + matK distinguish Eriophorum scheuchzeri subsp. scheuchzeri and E. scheuchzeri subsp. arcticum. The S. filiformis and O. borealis species complexes are both taxonomically problematic, and the differences in their barcode data may be a function of taxonomy that does not reflect evolutionary history [20].
The general lack of resolution of infraspecific taxa here parallels the findings of a barcoding study of Japanese pteridophytes that included ca. 40 additional infraspecific taxa and demonstrated that discrimination was lower when infraspecific taxa were considered as distinct species than when considered only at the species level [96], indicating that many infraspecific taxa could not be discriminated with the tested markers (the study did not indicate explicitly if any conspecific infraspecific taxa were discriminated). Supplementary markers such as ITS2 may be more useful in discriminating infraspecific taxa in the Arctic flora.
Infraspecific Genetic Variation
In a few species we detected considerable infraspecific variation that has not been reported previously in the literature. For example, the four sampled individuals of Lupinus arcticus S. Watson form two clusters, each with two individuals, based on the rbcL (two substitutions) and matK (five substitutions) data (Figure S19). We also found a deep genetic divergence in rbcL in Equisetum variegatum Schleich. ex F. Weber & D. Mohr, in which five substitutions define two clusters of individuals (Figure S17); we were not able to sequence matK in this genus. Infraspecific variation has not been reported in any Equisetum species previously, as phylogenetic studies have only sampled a single individual per taxon [197–199]. There is no variation in rbcL in any of the other Equisetum species that we sampled. Four additional rbcL sequences of E. variegatum have been published, from collections gathered in Churchill (GenBank accession no: JN965527 [109]), southern Ontario (HQ590086 [34]), Alaska (AY226134 [199]), and Japan (AB574691.1 [96]). The first three of these match the more common haplotype found in 14 individuals, and the Japanese sample matches the less common haplotype found in six individuals (data not shown). In both L. arcticus and E. variegatum there is no obvious geographical pattern to the observed variation, but it may represent phylogeographic variation or ancestral polymorphisms. Broader sampling from throughout the global ranges of these taxa is needed to properly characterize the observed variation from a geographical perspective. The variation in Equisetum is unlikely to be related to taxonomic problems with the circumscription of the species, as plants sampled here belong to the taxonomically stable circumboreal E. variegatum subsp. variegatum. A second subspecies not sampled here or elsewhere, E. variegatum subsp. alaskanum (A.A. Eaton) Hultén, is restricted to the Pacific coast in Alaska, British Columbia and Washington [200].
In a subset of taxa, most with problematic taxonomy, we detected infraspecific variation that may provide insight into the circumscription of taxa. Variation among individuals originally determined as Chrysosplenium tetrandrum Th. Fr. prompted us to re-examine the voucher specimens. We found a subset of these to be misidentified specimens of C. rosendahlii Packer, a species described from Somerset Island (Nunavut) [119] that was later reduced to synonymy or ignored [23,201], and more recently recognized again as a distinct species [202]. The barcode data distinguish C. tetrandrum, C. rosendahlii, and C. wrightii Franch. & Sav. (Figure S45). Variation in matK segregates the 21 sampled individuals of Stellaria longipes Goldie—a notoriously difficult polymorphic and polyploid species complex [20,203]—into two clusters (Figure S11). These clusters are distinguished by three characters, and there is further variation within each cluster, including a six base pair insertion shared by five individuals in the seven-individual cluster. We recognized all members of the complex sampled here as S. longipes, following recent treatments in North America (e.g., [25,204,205]), whereas other authors have recognized multiple species or infraspecific taxa in the complex [20,203]. Plastid variation apparently has not been studied within and among S. longipes and its closest relatives in North America. Additional study is needed to determine the origins of the plastid variation that we observe, and if it relates to the taxonomy of the species.
One individual of Astragalus eucosmus B.L. Rob., collected on Herschel Island, Yukon, has a matK haplotype distinct from other sampled individuals of the species, which cluster with another species, A. richardsonii E. Sheld. (Figure S19). The unique individual was previously identified as A. eucosmus subsp. sealei (Lepage) Hultén, a diploid amphi-Beringian taxon that has also been recognized as a distinct species, A. sealei Lepage (e.g., [20]). Astragalus sealei is included in A. eucosmus in other recent treatments (e.g., [25]), which we followed. Plastid variation has been detected previously in the widespread and tetraploid A. eucosmus [206], but that study did not sample subsp. sealei. Elven et al. [20] suggested that A. sealei is morphologically more similar to A. norvegicus Grauer, a Eurasian species, than it is to A. eucosmus. The variation in matK suggests that A. sealei and A. eucosmus may represent distinct plastid lineages and may support their recognition as distinct species, but broader samplings of both taxa and other putatively allied species, such as A. richardsonii and A. norvegicus, are needed to establish this.
We also detected considerable variation in species of Luzula (Figure S2). Some individuals of L. nivalis (Laest.) Spreng. are identical to individuals of L. arcuata subsp. unalachkensis (Buchenau) Hultén, while others have a distinct haplotype; individuals of L. confusa Lindb. cluster into two distinct groups; and the two sampled individuals of L. kjellmaniana Miyabe and Kudo each have unique haplotypes and do not cluster together. All of these species are classified in Luzula sect. Thyrsanochlamydeae Satake, along with L. subcongesta (S. Watson) Jeps., a Californian endemic [126]. The section was found to be non-monophyletic in phylogenetic analyses, and it has been suggested that the section or some of its species may be of hybrid origin [207–210]. These previous studies sampled only a single individual per taxon and did not detect infraspecific variation as we find here. Given the infraspecific variation uncovered in several Luzula species, sampling multiple individuals per taxon may be critical for resolving phylogenetic relationships among these and possibly other species in the genus.
Towards a Comprehensive DNA Barcode Library for Canadian Arctic Vascular Plants
We generated DNA barcode data for at least one of the core plastid loci for 490 vascular plant species, and for both loci for 418 species (88% of the total number of species sampled). These new barcodes represent nearly half of the some 1100 vascular plant species reported from the Canadian Arctic [20]. Many of the unsampled species are 'borderline' Artic taxa that barely extend into the region [20]. Barcode data for some species not sampled here have been generated from Churchill [109]. Our species sampling is comprehensive in the Canadian Arctic Archipelago. The Flora of the Canadian Arctic Archipelago [25] reported 341 species plus eight additional infraspecific taxa for the region. We have produced new barcode data for 316 (93%) of the vascular plant species known from this region (excluding from consideration the two Papaver taxa treated in the Flora due to a differing taxonomic treatment used here for the genus). Species from the Canadian Arctic Archipelago for which barcode data were not obtained are listed in Table S4. Continued taxonomic and geographical sampling is needed to complete the barcode reference library for Canadian Arctic vascular plants. Additional sampling is required for species with only one or a few individuals sampled, for species that have not yet been barcoded, and from poorly represented geographical regions, including northern Quebec and northern Labrador.
Species Resolution—A Closer Look at Barcode Variation Within Arctic Genera in the Context of Systematic Knowledge
Considering the ability of plastid barcodes to discriminate species in light of knowledge of their phylogenetic relationships and evolutionary history provides context helpful for understanding why some species are resolved by barcode loci and others are not. Barcoding studies at lower taxonomic levels (genus or family levels, for example) often consider patterns of variation in the barcode loci in the context of the systematics of their respective groups, whereas most broader barcoding studies have focused primarily on the overall ability of barcode loci to discriminate species in their data sets. The latter is unfortunate, as the large amounts of new data generated in such studies can provide important contributions to systematic knowledge in these groups, as we demonstrate here. Below we discuss a subset of our barcoding results in detail at the level of genus. In doing so, we highlight the ability of the core barcode loci to discriminate closely and distantly related species and place an individual among its closely related species in a species group. We also discuss putative identification problems, newly detected instances of possible hybridization and/or introgression, and the effects of widespread introgression on species identification with DNA barcodes. Given the large number of genera and species in the data set, we focus on a subset of genera in which we sampled few (two to four) species, and on the ten genera in the data set with the greatest number of species sampled (Puccinellia, Festuca, Poa, Pedicularis, Salix, Draba, Saxifraga, Ranunculus, Potentilla, Carex). We also discuss barcode variation in Arctic taxa of the dandelion genus (Taraxacum F.H. Wigg.) in light of previous studies that have demonstrated its complex and poorly understood patterns of plastid DNA variation, as well as barcode variation in the polyphyletic genus Minuartia.
Species Resolution in Genera with Few Species Sampled
The data set includes 53 genera with two to four species sampled for rbcL + matK. Among these species, discrimination with rbcL + matK ranges from 100% in 29 genera (Table 1) to 0% in 17 genera (Table 2). In the former group, phylogenetic work indicates that the few sampled species in many of these genera are not particularly closely related (e.g., Anemone L. [211], Arnica [194], Artemisia [193,212], Campanula L. [213], Cardamine L. [214], Kobresia [215], Pinguicula L. [216], Rhododendron L. [217], Stellaria L. [218], Tofieldia Huds. [219], Vaccinium L. [220]), and it is therefore unsurprising that the congeneric taxa can be distinguished with the core barcode loci. Complementary molecular data for some of the species have been generated in phylogeographic research. For example, the sampled species of Sagina L. (S. nivalis (Lindblom) Fr., S. caespitosa (J. Vahl) Lange) and Vaccinium L. (V. uliginosum L., V. vitis-idaea L.), all distinguished by rbcL and matK, have been shown to be similarly distinguished by variation in plastid intergenic spacers [221,222].
There are 21 genera with two or more species sampled in which there is no species resolution with rbcL + matK: 17 have two to four species, two have five species, one has 16 species, and one has 22 species (Table 2). Most of the indistinguishable species are closely related, and in several instances they are taxonomically problematic. There is either no variation in the plastid barcode loci among these species, or the variation in the core barcode loci is not consistent with species boundaries. Among these are several Asteraceae genera (Figure S5): Petasites, a genus with three northern taxa recognized here as two species, which have been variously treated as species or infraspecific taxa [129,130,223]; Tephroseris (Rchb.) Rchb., whose North American species are poorly defined and closely related [224,225]; and Antennaria Gaertn., whose taxonomy is complicated by polyploidy and apomixis [20,226]. There are several Poaceae genera with indistinguishable species (Figure S37): Agrostis L., a genus of morphologically similar species whose taxonomy is complicated by polyploidy and hybridization (e.g., [227]); Calamagrostis Adans., a large genus of polyploid taxa among which relationships are not clear [20,189]; Deschampsia P. Beauv., a genus that includes several closely related northern taxa that are morphologically and molecularly similar, and which may be best recognized as a single species [228]; Elymus L., a genus including several morphologically and molecularly similar northern taxa [229,230]; and Phippsia (Trin.) R.Br., a genus of two species with complicated taxonomic histories [231–233]. Other genera with indistinguishable species include Betula L. (Figure S6), a taxonomically difficult woody genus [20] in which the lack of discrimination among species here is consistent with other barcoding studies of the genus [34,234]; Papaver (Figure S33), a difficult genus in the Arctic with taxonomy complicated by hybridization and polyploidy [20,127]; Hippuris L. (Figure S35), an aquatic genus in which the three sampled taxa have been variously recognized as species or infraspecific taxa [20]; Stuckenia Börner (Figure S40), an aquatic genus with taxonomic problems among the northern species sampled [20,235]; and Cerastium L. (Figure S11), which includes several taxonomically difficult northern species with low levels of genetic variation [218,236,237].
Some of the congeneric species not distinguished by the barcode data are not closely related. For example, the polymorphic and widely distributed species Suaeda maritima (L.) Dumort. and S. calceoliformis (Hook.) Moq. [238] are each part of distinct, well-supported clades in a phylogenetic study based on ITS and two plastid intergenic spacer regions [239], whereas in our analyses these taxa have identical rbcL and matK sequences (Figure S3). In Primula, the three sampled taxa (P. borealis Duby, P. stricta Hornem., P. egaliksensis Wormsk.) are part of different well-supported major lineages in a phylogeny based on rapidly evolving plastid intergenic spacers [240], but the barcode data here fail to discriminate them, as haplotypes are shared among species (Figure S41). The inability of the core barcode loci to distinguish these Suaeda and Primula taxa may reflect identification problems, unresolved taxonomic problems with these species, or simply low variation in matK and rbcL compared with the more rapidly evolving plastid regions.
Puccinellia
Puccinellia is a taxonomically difficult grass genus with about 120 species, of which 12 occur in the Canadian Arctic [134,136]. We sampled all of these plus P. distans (Jacq.) Parl. from the adjacent boreal region, the amphi-Pacific/Beringian P. alaskana Scribn. & Merr., and P. hauptiana Scribn. & Merr. from Russia—a total of 15 species. Just four of 13 species with data for rbcL + matK are discriminated with the combined loci (Figure 7). Puccinellia species group into three main clusters, two of which are well-defined by their relatively long branches in the neighbour joining trees (Figure S48). One well-defined cluster includes P. pumila (Vasey) Hitchc., P. nuttalliana (Schult.) Hitchc., P. vaginata (Lange) Fernald & Weath., P. distans, and P. nutkaensis (J. Presl) Fernald & Weath. The second well-defined cluster includes P. tenella subsp. langeana (Berlin) Tzvelev, P. vahliana (Liebm.) Scribn. & Merr., P. phryganodes (Trin.) Scribn. & Merr., and P. alaskana. The remaining taxa plus one individual of P. pumila are intermixed; this group includes P. angustata (R. Br.) E.L. Rand & Redfield, P. arctica (Hook.) Fernald & Weath., P. bruggemannii T.J. Sørensen, P. andersonii Swallen, and P. banksiensis Consaul. The latter two groupings are similar in composition to major clades identified in recent systematic work on Arctic Puccinellia [137–140].
Only one of the recent studies [140] included P. nuttalliana, and none included P. vaginata or P. nutkaensis. Based on the plastid data here these taxa are closely related to each other and to most sampled individuals of P. pumila. The single individual of P. nuttalliana sampled by Consaul et al. [140] for nuclear ribosomal and plastid DNA was closely related to P. arctica and P. angustata, whereas our plastid data place the latter taxa and P. nuttalliana in different clusters. Individuals of P. pumila sampled previously for nuclear ribosomal and plastid DNA were part of a clade with P. arctica, P. banksiensis, P. andersonii, P. angustata, and P. bruggemannii [139,140]. By contrast, just one of the several individuals of P. pumila sampled here for rbcL + matK shows these affinities, with the remainder clustering with P. nuttalliana, P. vaginata, P. distans, and P. nutkaensis. Puccinellia pumila was considered to be part of the P. nuttalliana complex (also including P. nutkaensis) by Davis [241], a grouping supported by the barcode data for most individuals of the taxon. Given the variation in the species, the taxonomy and evolutionary history of P. pumila warrants further study. This is a good example of how barcode data can provide new insights into the taxonomy of under-studied or poorly known species.
The barcode data can readily place an unknown Puccinellia individual among a major group of species, which is helpful for narrowing the possibilities for an identification, but within each group multiple haplotypes are shared among most species. This may reflect the fact that many Arctic Puccinellia species are polyploids (8/13 species here, excluding P. distans, which is diploid and polyploid), a subset of which are known to have evolved from Arctic diploids and likely share plastid haplotypes with them [138,139]. In the first group, only P. nutkaensis (two individuals) and P. distans (one individual) have unique haplotypes based on the current sampling (Figure S48). In the second group, matK does not distinguish any taxa as multiple haplotypes [242] are shared among species within each of the two subclusters, and rbcL distinguishes just two taxa, P. phryganodes and P. tenella subsp. langeana (Figure S48).
The supplementary plastid regions psbA–trnH (Table S3, Figure S49) and psbK–psbI (Table S3, Figure S51) did not resolve any of the ten Puccinellia taxa sampled for these loci. The atpF–atpH intergenic spacer was more variable and distinguished four of nine Puccinellia species (Table S3, Figure S50), including three species that neither matK nor rbcL discriminates (P. alaskana, P. vahliana, P. arctica). Puccinellia tenella subsp. langeana, distinguished by rbcL, and P. nutkaensis and P. distans, distinguished by matK, were not sampled for atpF–atpH. They should be examined for this locus, as it may be the best plastid locus for resolution of Arctic Puccinellia species.
Festuca
We sampled all ten species of Festuca that occur in the Canadian Arctic [243]. Of these, only three-F. rubra L. s.l., F. baffinensis Polunin, and F. altaica Trin.—can be discriminated with the combined barcode loci (Figures 7, S52). Festuca rubra and F. altaica are not closely related to each other or to the remaining taxa [244], of which all but F. prolifera (Piper) Fernald are part of a broader group of arctic-alpine and non-Arctic taxa referred to as the Festuca ovina L. complex (sheep fescue complex) [245]. Members of this complex sampled here include F. baffinensis, F. brachyphylla, F. brevissima Jurtzev, F. edlundiae S.G. Aiken, L.L. Consaul & Lefk., F. hyperborea Holmen ex Fred., F. lenensis Drobow, and F. viviparoidea Krajina ex Pavlick subsp. viviparoidea, the latter a pseudoviviparous taxon.
Festuca baffinensis, F. brachyphylla, F. hyperborea, and F. edlundiae have been called the F. brachyphylla complex [122]. Festuca baffinensis is distinguished from the remainder of the complex by the barcode loci (Figure S52), a finding consistent with other morphological and molecular research [246,247]. Barcode variation among the remaining taxa is represented by a few haplotypes shared among species (Figure S52). Hybridization and/or introgression among the morphologically similar and closely related F. brachyphylla, F. edlundiae, and F. hyperborea [122,247] has been documented in Svalbard [246,247], and hybridization and introgression is also likely among these species in the North American Arctic, which may be the cause of their shared plastid barcode haplotypes. Festuca brevissima, a Beringian taxon that is distinct from other taxa in the North American F. ovina complex based on isozyme data [242], is not distinguished by the barcode data. Although two individuals of F. brevissima have a unique matK haplotype, matK in a third individual is identical to other members of the complex.
The plastid barcode data provide new insights into the putative origins of two viviparous Arctic Festuca taxa. Festuca viviparoidea may be of hybrid origin, possibly with F. brachyphylla and F. baffinensis as parental taxa [243,248]. If this is correct, we can infer that F. baffinensis is not the maternal parent, given that its plastid barcode haplotypes differ from those of other members of the F. brachyphylla complex, whose rbcL and matK sequences are identical to those of F. viviparoidea. An individual collected on the Belcher Islands and determined as F. prolifera—another vegetatively proliferating taxon—also clusters with the F. brachyphylla complex (Figure S52), a placement inconsistent with its putative evolutionary relationships. Festuca prolifera is considered part of the F. rubra complex based on morphological characteristics, and is readily distinguished from pseudoviviparous members of the F. ovina complex (F. viviparoidea, F. frederikseniae E.B. Alexeev) by its loosely cespitose habit and spreading rhizomes (vs. a densely cespitose habit), and straight leaves (vs. conspicuously curled leaves) [249]. Darbyshire and Pavlick [243] noted that viviparous plants from Greenland named F. villosa-vivipara (Rosenv.) E.B. Alexeev are similar to F. prolifera, and may be hybrids of F. rubra and F. frederikseniae, a pseudoviviparous taxon that occurs in Greenland, southern Quebec, and Newfoundland and Labrador. The plant from the Belcher Islands could be such a hybrid, or a hybrid between F. rubra and another member of the F. ovina complex; if so, its placement among members of the F. brachyphylla complex would not be unreasonable if the maternal parent were a member of the complex. Additional plastid and nuclear data from multiple individuals of F. prolifera, F. frederikseniae, F. villosa-vivipara, F. rubra and their putative hybrids are needed to determine their evolutionary relationships, as in recent research that has characterized relationships between F. ovina s.s. and the pseudoviviparous species F. vivipara (L.) Sm. in the United Kingdom [250].
Poa
Eleven species of Poa are known from the Canadian Arctic, including several boreal species that extend into the Low Arctic (P. leptocoma Trin., P. palustris L., P. paucispicula Scribn. & Merr., P. pseudoabbreviata Roshev.) [131,251]. We sampled all of these except P. leptocoma. We also sampled P. stenantha Trin., distributed in western North America. Five of the 11 Poa species sampled (P. abbreviata R.Br., P. alpina L., P. ammophila A.E. Porsild, P. pseudoabbreviata Roshev., P. paucispicula Scribn. & Merr.; Figure 7) can be distinguished with rbcL + matK (Figure S53). The closely related species P. abbreviata and P. pseudoabbreviata (Poa sect. Abbreviatae Nannf. ex Tzvelev [251]) have unique matK barcodes (Figure S53). Poa alpina is the only species of Poa sect. Alpinae (Hegetschw. ex Nyman) Stapf that occurs in Canada [251], and its barcodes distinguish it from all other Poa taxa sampled. The many sampled individuals of P. arctica R.Br. (including three subspecies) and P. pratensis L. (including three subspecies) are indistinguishable as they share several haplotypes (Figure S53). These species are closely related, morphologically similar, and are known to hybridize [187,251,252].
The supplementary plastid barcode regions do not distinguish any Poa species not distinguished by rbcL and/or matK. The psbA–trnH data distinguish P. abbreviata and P. alpina (Figure S49), the psbK–psbI data distinguish only P. alpina (Figure S51), and atpF–atpH data distinguish P. alpina and P. ammophila (Figure S50, Table S3).
Chloroplast capture is a well-known phenomenon in plants, and in such cases plastid barcode data alone will be problematic for discriminating species [34,39,42,51,253]. Previous work, based on restriction site and DNA sequence data, has documented two distinct chloroplast haplotypes in P. hartzii Gand. (Poa sect. Secundae V.L. Marsh ex Soreng): one unique to the species and one identical or similar to the dominant haplotype of the widespread and morphologically distinctive species P. glauca Vahl (Poa sect. Stenopoa Dumort.) [131,132,187]. The P. glauca haplotype in P. hartzii may have resulted from chloroplast capture of P. glauca plastid DNA via inter-sectional hybridization and introgression in the High Arctic [131]. We sampled individuals of P. hartzii known to contain either of the two haplotypes, in addition to samples of unknown haplotype, to demonstrate how such introgression is problematic for species identification via DNA barcoding.
The pattern of introgression is not evident in the rbcL tree, as rbcL sequences are identical in P. abbreviata, P. glauca, P. hartzii, P. stenantha Trin. and P. pseudoabbreviata (Figures S37, S53). In the matK tree, a subset of the sampled individuals of P. hartzii (and an identical individual determined as P. stenantha) is distinct compared with P. ammophila (also classified in Poa sect. Secundae) and all other taxa, whereas another subset of P. hartzii individuals is indistinguishable from individuals of P. glauca (Figures S37, S53). These latter individuals are easily identified as P. hartzii based on morphological characteristics, but they have plastid DNA introgressed from P. glauca. Given the presence of P. glauca plastid DNA in P. hartzii, these species cannot consistently be distinguished with plastid barcodes. We are able to interpret this introgressant pattern of plastid variation in light of a priori knowledge of chloroplast capture in P. hartzii, but in general when constructing barcode reference databases, instances of putative chloroplast capture are not usually known in advance or readily identifiable. When putative introgression is detected, factors such as sample misidentification, contamination, and incomplete lineage sorting also need to be considered to explain a putative incongruence between morphology and plastid lineage.
Our survey of plastid variation in P. hartzii also expands the distribution of the introgressed form of P. hartzii into the western Arctic. Gillespie et al. [132] found the introgressant P. glauca haplotype only in High Arctic populations of P. hartzii on Ellesmere Island and Axel Heiberg Island; none of the three populations sampled from Cambridge Bay, Victoria Island, had the P. glauca haplotype. We sampled three recent collections of P. hartzii from western Victoria Island (Ulukhaktok and Minto Inlet), and one of these (Gillespie et al. 10078) had the introgressed P. glauca haplotype. As barcode data accumulate and the number of individuals sampled per species increases, knowledge of the distribution of taxa with introgressed plastid DNA, as well as many new instances of putative introgression and chloroplast capture are likely to be documented, increasing our understanding of the prevalence of this phenomenon in the Arctic and elsewhere.
Pedicularis
Pedicularis is a globally distributed genus of hemiparasitic plants with more than 500 species [254]. We sampled the ten Pedicularis species that occur in the Canadian Arctic [20], and not more than 40% of these are discriminated by rbcL + matK (Figures 7, S54). A barcoding study of 88 species of Pedicularis from China, where the genus is most diverse, found rbcL + matK to distinguish 54–70% of species—depending on the criteria used for species discrimination—and suggested rbcL + ITS as the best two-locus barcode for the genus [62]. Most North American and Arctic species have not yet been included in phylogenetic studies of Pedicularis [254] and their relationships are unclear, but the barcode data suggest Arctic species are likely represented by several major lineages. There are deep genetic divergences among P. capitata Adams; P. flammea L.; P. verticillata L.; a P. albolabiata (Hultén) Kozhevn. and P. arctoeuropaea (Hultén) Molau & d.f. Murray cluster; a P. lapponica L. and P. labradorica Houtt. cluster; and a P. hirsuta L., P. langsdorffii Fisch. ex Steven, and P. lanata Willd. ex Cham. & Schltdl. cluster (Figure S54).
These divergent species and clusters are readily distinguished with the barcode data, but not all species are distinguishable within the clusters. Pedicularis albolabiata and P. arctoeuropaea, morphologically similar and closely related taxa recognized only recently at the species level [128], are not distinguished with the barcode data. In the P. lanata–P. hirsuta–P. langsdorffii cluster, the multiple individuals of each species also form clusters, consistent with their taxonomy, with the exception of one individual determined as P. langsdorffii that groups with P. hirsuta, and two specimens (Gillespie et al. 6105 from Axel Heiberg Island, and Gillespie et al. 5218 from Ellesmere Island) that are intermediate in morphology between P. hirsuta and P. langsdorffii with matK sequences identical to those of P. hirsuta (Figure S54). Porsild [22] also noted plants with morphologies intermediate between P. hirsuta and P. langsdorffii from northwest Greenland, and Ellesmere and Baffin Islands. These plants do not have a scientific name, and may represent hybrids (P. langsdorffii × P. hirsuta). If so, P. hirsuta may be the maternal parent, given its shared matK haplotype with the intermediate individuals. The individual determined as P. langsdorffii may also be a hybrid (or a misidentification), given its placement in the P. hirsuta cluster, or we may have detected another instance of introgression. Based on the current data there is no way to unambiguously distinguish P. hirsuta from putative hybrids for which it may be the maternal parental—a good example of how hybridization may negatively affect the success of barcoding with plastid markers. Pedicularis labradorica and P. lapponica are distinct from the rest of the genus. For the most part, individuals of these two species form sister clusters, but one individual of P. lapponica (Consaul et al. 3692 from the Belcher Islands, Nunavut) is identical in matK to P. labradorica. The specimen is correctly identified, thus this placement may reflect hybridization or introgression—which apparently have not been noted for these species—or contamination.
Salix
There are some 27 Salix species in the Canadian Arctic [255]. We sampled 20 of these plus one putative hybrid (S. arctica Pall. × S. polaris Wahlenb.) and three non-Arctic taxa (S. silicifolia Raup, S. stolonifera Coville, S. pseudomyrsinites Andersson). Discrimination with the core barcode loci fails remarkably in Salix, as no species can be discriminated (Figure 7). Although the Arctic species are morphologically diverse—ranging from dwarf, prostrate shrubs (e.g., S. herbacea L.) to several-meter-high tree-like bushes (e.g., S. alaxensis (Andersson) Coville)—variation in the barcode regions among the sampled species is very low (Figure S55). All but two individuals have identical rbcL barcodes. In matK there is slightly higher variation, but no matK haplotypes are unique to any species. These results are consistent with rbcL and matK data generated for willow species from Churchill [109] and Finland [234], which were similarly found to be indistinguishable. Kuzmina et al. [109] also found that multiple ITS2 haplotypes did not correspond to willow species boundaries. Low plastid variation among Arctic Salix species is also consistent with phylogenetic work in the genus, in which multiple species have been found to share identical plastid haplotypes [256–258].
Draba
Draba is a large and taxonomically complicated genus with extensive morphological variation, frequent hybridization, and polyploidy, and it is one of the largest genera in the Canadian Arctic [20,259]. Seventeen species are reported from the Canadian Arctic Archipelago [25], with additional species on the Arctic mainland [20,23]. Arctic Draba species are difficult to identify, in part because there are no up-to-date taxonomic keys for the genus in the Arctic or northern North America. Thus, some of our material may be misidentified. Of the 16 to 19 species we sampled, just one is discriminated by rbcL, and none are discriminated by matK and combined rbcL + matK data (Figures 7, S56). Identification of Draba species with DNA barcodes has also been problematic in other studies. For example, Yao et al. [56] examined 199 species of Draba in their study of ITS2 as a plant barcode, and found that just 27% of species could be discriminated—the second lowest level of success in their large study.
We find that most plastid haplotypes are distributed among two or more Draba species. In the rbcL tree, some individuals of D. juvenilis and D. glabella cluster together, while other individuals of these taxa share an identical haplotype with all other sampled taxa, except single individuals of D. crassifolia Graham and D. arctica, which each have unique haplotypes (Figure S8). We consider D. crassifolia to be discriminated by the rbcL data, but further sampling and matK data, which we were not able to obtain for this sample, are needed to confirm its putative distinctiveness. matK is more variable than rbcL in Draba, but most matK haplotypes are distributed among multiple species. No species can be distinguished with the matK data (Figure S8). In the rbcL + matK neighbour joining tree (Figures S8, S56) several clusters include individuals of one species (e.g., D. nivalis Lilj.) or a few species (e.g., individuals of D. corymbosa R. Br. ex DC. and D. lactea Adams; individuals of D. subcapitata Simmons, D. simmonsii Elven & Al-Shehbaz, D. pilosa Adams ex DC., and D. micropetala Hook.; individuals of D. glabella Pursh, D. juvenilis Kom., and D. arctica J. Vahl), but other individuals of most of these species share a haplotype with other species. The exceptions may be due to misidentification and/or hybridization, and further investigation combined with a better understanding of the taxonomy is necessary to determine if species and species groups can be resolved.
Saxifraga
We sampled all of the 14 species of Saxifraga that occur in the Canadian Arctic [23,25], and 69% of these are distinguished with rbcL + matK—the second highest species resolution among the ten largest genera sampled (Figure 7). The clustering pattern of Saxifraga species, based on the barcode data, is similar to evolutionary tree topology in a phylogenetic study based on matK and ITS data that included nine of the species sampled here [260]. In the current sampling only the species pairs S. cernua L. and S. radiata Small, and S. bronchialis L. and S. tricuspidata Rottb., are indistinguishable with the combined data (Figure S57). Saxifraga cernua, a heterogeneous high polyploid that likely is a complex hybrid species, and S. radiata, which includes diploid and low polyploid individuals, are part of the "Saxifraga sibirica L. aggregate", and transitional forms and/or hybrids between the species have been documented in various parts of their ranges [20]. This may be the reason for a shared haplotype among the two sampled individuals of S. radiata and one individual of S. cernua. Saxifraga bronchialis (treated as S. funstonii (Small) Fedde in Elven et al. [20] and S. bronchialis subsp. funstonii (Small) Hultén in Brouillet and Elvander [261]) and S. tricuspidata are closely related and part of the S. bronchialis complex, a group that requires additional taxonomic research [261]. matK failed to amplify for S. rivularis, a tetraploid species that is distinct from the closely related diploid S. hyperborea [262]. Saxifraga hyperborea is thought to be the maternal parent of S. rivularis, and matK sequences generated for these taxa from Svalbard differed by a single substitution [263]; they cannot be discriminated with the rbcL data presented here.
Ranunculus
Ranunculus is represented in the Canadian Arctic by some 13 species, the exact number depending on how some taxa are circumscribed [20,264]. We sampled 12 species for rbcL and 11 for matK and rbcL + matK. Seven of 11 species (63%) are resolved with the combined barcode data (Figure 7). In the neighbour joining trees, Ranunculus species cluster into two main groups (Figure S58) that generally correspond to two clades identified in several phylogenetic studies [265–269]. One group comprises the water buttercups and includes two subclusters: one of R. confervoides (Fr.) Fr., R. subrigidus W.B. Drew, and R. aquatilis var. diffusa With. (Ranunculus sect. Batrachium DC.); the other of R. hyperboreus and R. gmelinii DC. (Ranunculus sect. Hecatonia (Lour.) DC.). The second group includes R. sulphureus Sol., R. allenii B.L. Rob., R. sabinei R.Br., R. pygmaeus Wahlenb., R. nivalis L., and R. arcticus Richardson. This group is classified as Ranunculus sect. Auricomus Tamura, distributed in Arctic-circumpolar to temperate mountain regions in the northern hemisphere [269]. All of the sampled taxa in this cluster are distinguished by the barcode data. Ranunculus allenii, a northeastern North American endemic [20], has not previously been included in phylogenetic analyses. This species clusters near, but is distinct from, R. pygmaeus, supporting its inclusion in Ranunculus sect. Auricomus.
Our data support a close relationship between R. gmelinii and R. hyperboreus, as the sampled individuals of these species are intermixed and indistinguishable from each other in a cluster that is part of a larger cluster of aquatic taxa (Figure S58). However, the affinities of R. gmelinii are contradictory in other studies. Ranunculus gmelinii subsp. gmelinii is the sister group of the terrestrial clade based on combined matK/trnK and ITS data in Paun et al. [265] and Hoffmann et al. [266] (the same sequences were used in both analyses). Hoffman et al. [266] also included matK and ITS data for a different infraspecific taxon of the species, R. gmelinii subsp. purshii (Richardson) Hultén, which they found to be closely related to R. hyperboreus Rottb. Other studies used these same R. gmelinii subsp. gmelinii sequences plus data for an additional plastid region (psbJ–petA), and these analyses placed the taxon firmly among other aquatic taxa—as do our data—either as the sister group of R. hyperboreus (parsimony analyses [268]) or as part of a polytomy with other aquatic species [267]. The reason for the discordant placement of R. gmelinii subsp. gmelinii among these studies is unclear, since all used the same matK/trnK and ITS data for the taxon; an additional plastid region is unlikely to affect its position in the trees. Curiously, there are no comments on these differences in the more recent studies [267,268]. As in Scott [270] and Whittemore [264] we do not recognize infraspecific taxa in R. gmelinii, whereas Elven et al. [20] recognize subspecies gmelinii and purshii, as in the phylogenetic studies. Among the other sampled taxa in the water buttercup cluster, only R. confervoides is distinguished. Ranunculus subrigidus and R. aquatilis var. diffusa (=R. trichophyllus Chaix in Elven et al. [20]), which are sometimes considered to be conspecific (e.g., [264]), have identical barcodes.
Potentilla
The taxonomy of Potentilla in the Arctic is notoriously difficult, complicated by extensive hybridization, polyploidy, agamospermy, phenotypic plasticity, and varying taxonomic viewpoints [20]. Aiken et al. [25] reported 11 species from the Canadian Arctic Archipelago, and some 33 species have been reported for the North American Arctic (excluding western Alaska and western Greenland) [20]. We sampled 13 species for rbcL and 12 species for rbcL + matK; our sampling focused primarily on species from the Canadian Arctic Archipelago. In the barcode data there is a deep divergence between P. anserina (Wormsk.) Rydb. and the remainder of the Potentilla taxa sampled (Figure S59). This is consistent with the phylogenetic position of P. anserina in a clade that is the sister group of a clade comprising most species of Potentilla (i.e., Potentilla s.s.) and several (non-Arctic) segregate genera in plastid analyses [271,272]. Potentilla anserina has been treated in a separate genus, Argentina Hill [25,273], a circumscription that is consistent with the phylogenetic evidence, although recently some authors kept the species in Potentilla (e.g., [20,272]). If recognized, Argentina can be discriminated by the core barcode loci, as is the case for most genera here, including all Rosaceae genera (Figure S43).
Among Potentilla s.s. species, P. biflora Willd. ex Schltdl. is distinguished from the rest of the sampled taxa (Figure S59), consistent with its known distant relationship with a large Potentilla clade—the "Potentilla core group" of Dobeš and Paule [271] and the "Argentea clade" of Topel et al. [272]—that includes many taxonomic series/sections, including those in which all other sampled Arctic taxa are classified. Among these taxa there are five rbcL + matK haplotypes: one unique to P. bimundorum Soják and four distributed among the remaining taxa that are present in 40, 18, 10, and one individual, respectively (Figure S59). Most of the taxa sampled are part of Potentilla sect. Niveae (Rydb.) A. Nelson, a taxonomically challenging group in which species delimitation is problematic [274–276]: P. subvahliana Jurtzev, P. uniflora Ledeb., and P. villosa Pall. ex Pursh (three of five species that are part of the P. uniflora aggregate); P. nivea (one of two species comprising the P. nivea aggregate); P. arenosa (including P. chamissonis Hultén, a synonym of P. arenosa subsp. chamissonis (Hultén) Elven & d.f. Murray); and P. vahliana and P. subgorodkovii Jurtzev (species of putative hybrid origin from crosses between the P. uniflora aggregate and the P. nivea aggregate [20]). A few taxa are part of other sections: P. hyparctica Malte is included in Potentilla sect. Aurea (Rydb.) Juz; P. bimundorum and P. pulchella R.Br. are included in Potentilla sect. Pensylvanicae Poeverl; and P. rubricaulis is included in Potentilla sect. Rubricaules (Rydb.) A. Nelson. Our sampling also includes several presumed intersectional hybrids (Potentilla sect. Nivea × Potentilla sect. Pensylvanicae) that are taxonomically problematic [20,30].
None of these sections has been recovered as monophyletic in phylogenetic analyses [271,272], and within Potentilla sect. Niveae only the P. uniflora aggregate has been recovered as a monophyletic group, based on trnS/G and trnL–F plastid data [272]. Dobeš and Paule [271] suggested that the inability to recover any of the sections as clades may be due to incomplete lineage sorting, hybridization among sections and series, low molecular variation, or problems with the classification. Hybridization among members of Potentilla sect. Nivea in northern North America has been documented with molecular data [276]. All of these factors may also contribute to the shared rbcL and matK barcodes found in these taxa.
Although most aspects of species relationships in Potentilla are unresolved in phylogenetic studies, there is branch length variation in the phylogenetic trees generated from plastid intergenic spacers and introns [271,272], suggesting that rapidly evolving plastid regions may be more informative than the rbcL and matK DNA barcodes for species identification in the genus. However, haplotypes in other plastid regions are also likely to be shared among species, as in the core barcode regions. Microsatellites have recently been developed for species in the Potentilla core group with high cross-transferability across the clade [277]. These may be useful for characterizing hybridization, species limits and genetic diversity in Arctic species of Potentilla, which in turn may provide insights into the distribution of plastid lineages among the Arctic taxa.
Carex
Carex is the most species-rich genus in the Canadian Arctic, and the genus for which we sampled the greatest number of species. Carex also has the highest species resolution among genera with multiple species (i.e., >10) sampled (Figure 7), counter to the general trend observed of decreasing resolution with increased species sampling. matK alone distinguishes 82% of sampled species (Figure S60), and if the three sampled species of the genus Kobresia—which is phylogenetically nested within Carex (e.g., [215,278,279])—are considered with Carex, matK resolves 84% of species (Figure S13). In Carex (and more broadly in Cyperaceae), matK is much more variable than rbcL, which alone resolved only some 18% of Carex species. Not unexpectedly, these results are very similar to the findings of Le Clerc-Blain et al. [108] who characterized the success of multiple plastid markers for barcoding sedges (Carex and Kobresia) of the Canadian Arctic Archipelago, as there is major overlap in the species they studied and those sampled here. They found that matK alone could distinguish some 85% of 23 species, and rbcL resolved 67% of 12 species (the rbcL data set was incomplete). The sampling in the current study includes new barcode data for all of the Carex and Kobresia species studied by Le Clerc-Blain et al. [108] as well as new data for 11 species previously unsampled for matK and 23 Carex and two Kobresia species previously unsampled for rbcL. Despite a 47% increase in the number of species (from 23 to 34) in the matK data set, the percent species resolution is similar to the earlier analysis with just 23 species.
The high species resolution in Carex in the Canadian Arctic is partly a function of the numerous distant lineages represented in the Arctic flora. One major cluster in the neighbour joining tree corresponds to Carex subg. Vignea [278,279] (Figure S61). Phylogenetic studies of Carex subg. Vignea have variously included a few of the Arctic species sampled here, which are classified in diverse sections, including C. gynocrates Wormsk. ex Drejer (Carex sect. Physoglochin Dumortier [280]), C. chordorrhiza Ehrh. ex L. f. (rbcL only, Figure S13; Carex sect. Chordorrhizae (Heuffel) Meinshausen [281]), C. maritima Gunnerus (Carex sect. Foetidae (Tuckerman ex L. H. Bailey) Kükenthal [282]), C. diandra Schrank (Carex sect. Heleoglochin Dumortier [283]), and C. canescens L. and C. lachenalii Schkuhr (Carex sect. Glareosae G.Don [284]). These sectional taxa comprise different lineages [285–288], and each of these species is distinguished by the barcode data. Carex marina Dewey and C. ursina Dewey, also classified in Carex sect. Glareoseae, are also distinguished. They have not been included in phylogenetic studies, thus their relationships with each other and other taxa in the section are not known. There is particularly deep divergence in the barcode data between C. diandra and the other sampled subg. Vignea species, consistent with their distant relationships [285].
Another major cluster in the neighbour joining tree corresponds to a clade of unispicate taxa (the Core Unispicate Clade [278,279]) found in phylogenetic studies (Figure S61). This clade includes species of Carex and Kobresia (e.g., [215,289]), and the five species of the clade sampled here are all distinguished by the barcode data (C. rupestris All., C. nardina Fr., K. simpliciuscula (Wahlenb.) Mack., K. myosuroides (Vill.) Fiori, K. sibirica (Turcz. ex Ledeb.) Boeck.).
The majority of the Carex species sampled are part of the Core Carex Clade [278,279]. Carex membranacea Hook., C. saxatilis L., and C. rotundata Wahlenb. are part of Carex sect. Vesicariae (Heuffel) J. Carey [290]. Le-Clerc Blain et al. [108] found that C. membranacea and C. saxatilis were distinguished by a single nucleotide polymorphism, consistent with the current data. They did not sample C. rotundata, which we find to be distinguished from C. membranacea by one matK substitution and from C. saxatilis by two matK substitutions (Figures S60, S61). A recent phylogenetic study of Carex sect. Vesicariae in Siberia based on matK, atpF–atpH, ITS2 and the nuclear hsp90 gene, found that C. saxatilis and C. rotundata fall in different major clades [291]. Carex membranacea was not included in this study. Our matK sequences and those generated by Le Clerc-Blain et al. [108] for C. membranacea are identical to those of C. rostrata Stokes in the study of Siberian plants (data not shown), and atpF–atpH sequences [108] are identical to those of several species in the clade that includes C. rostrata and C. rotundata (data not shown).
Carex aquatilis Wahlenb. and C. subspathacea Wormsk., both classified in Carex sect. Phacocystis, share two matK haplotypes. These species are closely related, as evidenced by a phylogenetic analysis that found a clade comprising a paraphyletic C. aquatilis and a sublineage including the maritime species C. subspathacea, C. paleacea Schreb. ex Wahlenb. and C. ramenskii Kom. (the latter two species are also found in the Canadian Arctic but not sampled here). Carex subspathacea hybridizes with C. aquatilis and other species [292], and this may be reflected in their shared barcodes. Carex bigelowii Torr. ex Schwein., a taxonomically problematic species (e.g., [293]) that is also part of Carex sect. Phacocystis, is more distantly related to C. aquatilis and C. subspathacea [294,295]; this is reflected in its distinct matK barcode. Carex spectabilis Dewey and C. podocarpa R.Br. ex Richardson (two of 11 species in Carex sect. Scitae Kükenthal [296]) cluster together but have distinct matK haplotypes. Carex holostoma Drejer and C. norvegica Retz. (Carex sect. Racemosae G. Don [297]) are distinguished, but their evolutionary relationships are not known as few of the ca. 60 species of Carex sect. Racemosae have been included in phylogenetic studies. Carex bicolor Bellardi ex All. and C. garberi Fernald (Carex sect. Bicolores (Tuck. ex L.H. Bailey) Rouy [298]) have distinct barcodes, as do C. vaginata Tausch and C. livida (Wahlenb.) Willd. (Carex sect. Paniceae G. Don [299]).
Other species classified in multiple sections are also distinguished with the barcode data, including C. rariflora (Wahlenb.) Sm. (Carex sect. Limosae (Heuffel) Meinshauser [300]), C. scirpoidea Michx. (Carex sect. Scirpinae (Tuckerman) Kükenthal [301]), C. concinna R.Br. ex Richardson (Carex sect. Clandestinae G.Don [302]), C. supina Willd. ex Wahlenb., and C. glacialis Mack. (Carex sect. Lamprochlaenae (Drejer) L.H. Bailey [303]). Carex krausei Boeck. and C. capillaris subsp. fuscidula (V.I. Krecz. ex T.V. Egorova) Á. Löve & D. Löve (Carex sect. Chlorostachyae Tuckerman ex Meinshauser [304]), taxa sometimes considered conspecific but currently recognized as species (see 30), are distinguished by a substitution in rbcL (their matK sequences are identical). Carex supina and C. glacialis have not been included together in phylogenetic analyses thus their relationships are not known.
Carex petricosa Dewey, C. fuliginosa subsp. misandra (R. Br.) Nyman and C. atrofusca Schkuhr are classified in Carex sect. Aulocystis Dumortier [305]. Each is readily distinguished by the barcode data, and each species clusters in distinctly separate parts of the neighbour joining tree, suggesting that one or all of these may be misclassified in Carex sect. Aulocystis. Ball and Mastrogiuseppe [305] noted that C. atrofusca may be misplaced to section, as it is the only species in the section with papillose perigynia, and the barcode data support this possibility. Hendrichs et al. [306] included C. fuliginosa and C. atrofusca in their phylogenetic study, and found their relationships to be unresolved with respect to each other and two other sampled species of Carex sect. Aulocystis. Carex petricosa has not been included in any phylogenetic studies to date. A proper phylogenetic analysis including these taxa and other species of Carex sect. Aulocystis in the context of the Core Carex Clade is needed to resolve their affinities.
Taraxacum
Taraxacum is a complicated agamic complex of sexual diploid (10% of species) and apomictic polyploid (90% agamospecies) taxa, in which over 2000 species (microspecies) have been described [307]. Species are classified into 47 sections based on morphological and geographical criteria [308]. A few sections include only sexual diploids, and most include sexual diploids and apomictic polyploids [307,309,310]. Taraxacum sections are considered to be either ancestral (mostly sexual, diploid, ancestral morphology) or derived (polyploid or diploid, asexual or sexual, derived morphology) [307], the latter thought to have originated from the ancestral taxa via hybridization and extensive reticulation [310]. Ancestral sections are geographically restricted, occurring in mostly unglaciated regions in west central Asia and the Mediterranean, whereas derived sections are more widespread occurring on coastal Eurasia, major Eurasian mountain ranges and arctic-alpine regions. Apomicts are thought to have originated via autopolyploidy or hybridization among sexual species, although the ancestors of most agamospermous taxa and their evolutionary origins are unknown [310] and it is probable that some or all of the parental lineages are themselves of hybrid origin [311]. Little is known about the interrelationships among sections and their evolutionary histories [307,309,311].
Several studies of chloroplast DNA have shown that multiple plastid haplotypes are shared among sections and species of Taraxacum (i.e., these sections and species are paraphyletic or polyphyletic), based on variation in trnL–F (sequence and restriction fragment length polymorphism data) and psbA–trnH (sequence data) [309,311,312]. In a large study of plastid variation in Taraxacum including 237 species from Europe, Asia, and North America, Witzell [309] found that several morphologically defined sections often had multiple shared haplotypes from up to seven plastid DNA lineages. Similarly, Majeský et al. [313] found five trnL–F haplotypes in nine microspecies of Taraxacum sect. Taraxacum (=T. officinale F.H. Wigg. aggregate), of which one was found in seven taxa and one was unique to one microspecies. Based on a lack of congruence between the morphologically defined sections and the plastid data, Kirschner et al. [311] suggested that either Taraxacum supraspecific taxonomy is wrong, or the plastid gene tree does not reflect the extensive reticulation that has occurred in the genus. Witzell [309] noted that the maternal lineages in most sections are polyphyletic as a result of repeated hybridization. In addition to shared plastid haplotypes among sections, King [312] found infraspecific plastid variation with restriction enzymes in non-native asexual microspecies sampled from North America (non-Arctic) and Europe, suggesting these microspecies may have multiple origins. Witzell did not find any infraspecific variation in 12 apomicts in which only two individuals were sampled.
Aside from recently published barcode data for Taraxacum species from Churchill, on which Kuzmina et al. [109] did not comment, genetic variation within and among Canadian Arctic dandelions has not previously been studied. The species sampled here include seven native and one introduced species classified in four sections: T. officinale (introduced; Taraxacum sect. Ruderalia Kirschner, H. Øllg. & Štepánek), T. ceratophorum (Ledeb.) DC. (Taraxacum sect. Borealia Hand.-Mazz.), T. lapponicum Kihlm. ex Hand.-Mazz. (Taraxacum sect. Spectabilia Dahlstedt), T. carneocoloratum A. Nelson, T. holmenianum Sahlin, T. hyparcticum Dahlst., T. phymatocarpum J. Vahl, and T. scopulorum (A. Gray) Rydb. (Taraxacum sect. Arctica Jurtzev) [314]. In the treatment of Taraxacum for the Flora of North America, which we followed, Brouillet [314] recognized 15 species circumscribed broadly (compare this, for example, with Porsild and Cody [23] who recognized 16 species in the continental Northwest Territories and Nunavut). Variation in rbcL and matK among the multiple Taraxacum individuals and taxa sampled here is low, and haplotypes are shared among species (Figure S5). In rbcL, we detect four haplotypes: one found in most individuals, one in an individual of T. lapponicum, one in an individual of T. carneocoloratum, and one in one individual each of T. ceratophorum and T. hyparcticum. In matK, we detect four common haplotypes found in 21, six, nine, and nine individuals, respectively, with each group including two to six taxa. A single individual in two of these groups has a unique haplotype (one of these is from the only individual of T. officinale sampled), and two individuals in the largest group each have unique haplotypes.
matK has not been sequenced extensively for dandelions and therefore we cannot compare our sequences with those from other Taraxacum lineages. Nevertheless, discovery of multiple haplotypes distributed among three of the four sampled sections of Taraxacum is fully consistent with the previous studies that have detected this same plastid DNA pattern across an even greater number of species and sections [309,311,312]. Furthermore, our finding of multiple plastid haplotypes within species (infraspecific variation) is consistent with the results of King [312]. There are several possible explanations for the plastid patterns observed. The infraspecific variation in Arctic Taraxacum may reflect multiple origins of species and, by direct implication, multiple origins of the sections. Alternatively, the infraspecific variation may be a function, wholly or in part, of incorrect taxonomy. This may be attributed to incorrect identification of the material that we studied, a subset of which was difficult to identify, or may be a function of the broad species circumscriptions currently in use in North America, which may not accurately reflect the species' evolutionary histories. For example, T. ceratophorum and T. lapponicum have both been subdivided into numerous microspecies [314], and infraspecific variation may be detected if the microspecies have independent origins. We did not attempt to identify the samples to putative microspecies, as there is no satisfactory treatment available. Several authors have cautioned that correct identification is critically important when attempting to characterize genetic variation and gene flow in Taraxacum apomicts [309,315].
Another explanation for the observed pattern is that a subset of the Arctic taxa may be parental to some or all of the other taxa. Five of the seven native Arctic taxa are exclusively polyploids and their origins are unknown, T. holmenianum is diploid and putatively sexual, and a range of ploidy levels has been documented in T. ceratophorum. Taraxacum ceratophorum reproduces sexually in Colorado, where all populations studied were diploid [316], but its mode of reproduction in the Arctic is unknown. Three of the four main plastid haplotypes are found in individuals of T. holmenianum and in other taxa. The origin of the infraspecific plastid variation in this diploid taxon is unknown. Taraxacum holmenianum may be one of the parental taxa of the polyploid species, perhaps representing the maternal lineage from which they have obtained their plastid haplotypes. Plastid variation has similarly been found in a sexual species from Poland (T. tenuifolium Koch) [311]. This putative scenario does not explain the presence of the same shared haplotypes in T. ceratophorum, which is morphologically distinct from T. holmenianum and part of a separate section. Whatever the origins of the plastid variation, the multiple shared haplotypes found in multiple species here and elsewhere suggest that plastid DNA barcodes are of little use for species identification in dandelions.
Minuartia
Our data are consistent with previous studies that found the genus Minuartia to be polyphyletic [218,317], as two clusters of species are widely separated in our neighbour joining trees. One cluster includes M. biflora (L.) Schinz & Thell. and M. arctica (Steven ex Ser.) Graebn., and the other includes two subclusters: one comprises M. rubella (Wahlenb.) Hiern, the other comprises the closely related M. elegans (Cham. & Schltdl.) Schischk., M. stricta (Sw.) Hiern, and M. rossii (R.Br. ex Richardson) Graebn. (Figure S11) [318]. Based on the considerable genetic distance between these subclusters, they are probably not closely related. The polyphyletic Minuartia has not yet been re-classified pending further sampling of the genus [317]. Arctic species will likely be placed in two or three different genera, which the current barcode data will distinguish.
Our sampling in Minuartia has revealed the misidentification of a sequence used in phylogenetic analyses of Caryophyllaceae [218,317]. The M. rossii matK sequence (FJ404849) in these studies was generated from the collection Gillespie et al. 6726 (CAN) [317], which we sequenced independently. Our matK sequence is identical to the published sequence and falls in a cluster with individuals of M. biflora (Figure S11), similar to one of the phylogenetic studies [218]. Other individuals of M. rossii, however, are part of a distant cluster that also includes M. stricta and M. elegans (Figure S11). Re-examination of the Gillespie et al. 6726 voucher specimen revealed the original identification to be an error; the correct identification for the specimen is M. biflora.
DNA Barcode Data and the Post-Glacial Origins of the Arctic flora
Molecular data from several arctic-alpine species have been studied to identify potential glacial refugia and to reconstruct the post-glacial origins of the Arctic flora (reviewed in 319). Many of these studies have been based on plastid sequence data from intergenic spacer regions, which are thought to evolve more rapidly than protein coding genes. Variation in plastid protein-coding genes such as matK and rbcL has not thus far been explored in phylogeographic studies of arctic-alpine species. Nevertheless, these genes may be sufficiently variable to also provide insights into the historical biogeography of Arctic and other taxa. If so, sequencing of the core barcode loci for plant taxa from throughout their geographical ranges may provide data informative for this field of research. The data we generated for the Arctic flora, in combination with recent data from other barcoding studies, provide an opportunity to see if the geographical distribution of infraspecific variation in the core barcode loci in arctic-alpine species correlates with geographically structured plastid variation found previously in these taxa.
Oxyria digyna (L.) Hill
Oxyria digyna is an arctic-alpine species widely distributed across the northern hemisphere in which six major plastid lineages have been identified, based on sequence data of the trnH–psbA and trnT–trnL intergenic spacers [320,321]. The seven individuals sampled by Allen et al. [321] from the Canadian Arctic were part of a broader Arctic clade that ranges from Greenland to eastern Siberia, and the individuals they sampled from the Northwest Territories and Nunavut—the range of the specimens we sampled here—all had an identical haplotype ("haplotype 3"). We found no variation in rbcL (n = 6) or matK (n = 8) among the plants we sampled (Figure S39). This is not unexpected, as we likely sampled only one of the plastid lineages in the species. Broader geographical sampling is needed to determine if the known plastid lineages can be detected in rbcL and matK.
Vaccinium uliginosum L
Alsos et al. [222] identified three major plastid lineages in the widespread blueberry species V. uliginosum that correspond to geography: a Beringian lineage, an amphi-Atlantic lineage, and a widespread arctic-alpine lineage. Nearly all their sampled specimens from the Canadian Arctic were part of the globally widespread arctic-alpine lineage. However, two populations from Churchill were part of the amphi-Atlantic lineage. There is no variation in rbcL and matK among our Canadian Arctic samples and one boreal sample from Yellowknife, NT (Figure S18). However, V. uliginosum matK sequences from a Churchill (Manitoba) collection (JN966729.1 [109]) and a St. John's (Newfoundland and Labrador) collection (AF419717.1, vander Kloet 217995 [322]), which are identical, differ from our Arctic matK sequences (data not shown). These two matK haplotypes may represent the amphi-Atlantic and arctic-alpine lineages, respectively. (The provenance of the vander Kloet 217995 collection in Powell and Kron [322] is given only as 'circumboreal'. The specimen could not be located in the E.C. Smith Herbarium (ACAD), but the collector's field notes indicate the place of collection as Signal Hill, St. John's, 17 Sep 1995 (R. Newell, personal communication, 28 Jan 2013)). rbcL sequences for the species have been published for collections from Churchill (JN966056.1 [109]) and Sweden (AF421107.1 [323]). These are identical to those we generated from the Canadian Arctic. No variation in rbcL in V. uliginosum has thus far been detected.
Saxifraga oppositifolia (L.)
Considerable research has been conducted on the phylogeography of purple saxifrage (Saxifraga oppositifolia), a widely distributed species that occurs throughout the global Arctic and further south in European and North American mountain ranges [324]. Two distinct lineages in the species have been found in studies of RFLPs, plastid sequence data and AFLPs [324–327]. Abbott et al. [325] called these the "Eurasian clade" (the "European-centred clade" of Winkler et al. [327])—from Newfoundland and Labrador and Greenland, across the Atlantic Arctic to the Taymyr Peninsula in northern Siberia, and south in the Pyrenees, Alps, and Tatra Mountains—and the "American-Beringian clade" (the "North American clade" of Winkler et al. [327])—from northern Greenland, west through Beringia to Central Asia and into southern and south-eastern European mountain ranges. Sampling from Canada in these studies was sparse [326,327]; the Eurasian clade is known only from Newfoundland and Labrador and southern Baffin Island, and the American-Beringian clade is known from the High Arctic and south-western Canada. Given the distribution of known populations of the American-Beringian clade, it is reasonable to assume that this lineage extends throughout much of the Canadian Arctic, but its precise limits are unclear. Likewise, the geographical extent of the Eurasian clade in Canada is not known.
The current barcode data may provide some insight into this. There are two haplotypes represented in the 11 rbcL sequences we obtained for S. oppositifolia: one in an individual from the Lower Savage Islands, Nunavut (southeast of Baffin Island), and one in the remaining ten individuals, which were all collected in the western Arctic and High Arctic (Ellesmere Island) (Figure S45). Sequences from Churchill, Manitoba, Canada (JN965986) [109] and the United Kingdom (JN891054.1) [98], sampled as part of barcoding studies, represent the former rbcL haplotype (data not shown). The two rbcL haplotypes in the North American Arctic may represent the two major lineages of S. oppositifolia, as they generally correspond to the distributions of the two clades as supported by other plastid markers. Indeed, all plants sampled from the United Kingdom are part of the Eurasian clade, and the putative presence of this lineage on the Savage Islands is consistent with current knowledge of its extent in eastern Canada [324] (the Savage Islands are geographically intermediate between the Baffin Island and the Newfoundland and Labrador populations of the Eurasian clade). If this rbcL haplotype represents the Eurasian clade, the distribution of the lineage would be extended considerably westwards in Canada to Churchill, where the haplotype is also present. Broader rbcL sampling is needed to confirm whether the variation detected here reflects the previously detected lineages, ideally of the samples used in previous work. Corresponding data for matK are not available as we failed to recover matK sequences from all but one sample of S. oppositifolia, as noted previously.
Conclusions
We have generated new plastid DNA barcodes for nearly half of the vascular plant species in the Canadian Arctic ecozone, and 93% of the species in the Canadian Arctic Archipelago. Although the core plastid barcode loci distinguish just over 50% of the sampled Arctic species, this figure does not convey the full utility of a comprehensive DNA barcode reference library for facilitating species identification. Almost all species can readily be assigned to their correct genus, a level of identification that may be sufficient for some purposes (e.g., [107,328]), and in many families the core plastid loci—even matK alone—will routinely place an unknown taxon amongst its most similar congeneric species, with the exception of some genera (e.g., Draba, Potentilla, Salix, Taraxacum) where levels of variation are too low to discriminate species, and/or the distribution of barcode haplotypes among individuals is not consistent with species boundaries as they are currently understood.
The barcode data facilitated identification of taxonomic and identification problems, as the local placement of misidentified specimens in neighbour joining trees was often insightful in helping us make correct morphology-based identifications, even when several closely related taxa shared barcode haplotypes. Detailed examination of the barcode data in a subset of taxa indicated, not unexpectedly, that the ability of the core plastid barcode loci to discriminate species is closely related to the evolutionary relationships of sampled taxa. Such in-depth analysis of barcode data is not included in many barcoding studies. By considering variation in a few exemplar species, we have demonstrated that plastid barcode data may provide major new insights into the biogeographical history of the Arctic flora, when barcode data are available from throughout species' global geographical ranges.
As only a small fraction of the planet's plant diversity has been sequenced for both of the core plastid barcoding loci, the upper limit for species resolution on a global level with these markers is not yet known. Nevertheless, given the low resolution for these markers in studies that sampled a large number of taxa, including the current one, it is clear that additional loci are necessary to improve discrimination of plant species with DNA barcodes, as recommended by the CBOL Plant Working Group [329].
Supporting Information
Voucher specimen information, including the scientific names of taxa sampled, locality information, collection dates, collectors and collection numbers, herbarium accession numbers, and GenBank accession numbers.
(XLSX)
Species with two or more infraspecific taxa sampled, and the ability of rbcL, matK, and rbcL + matK to resolve the conspecific infraspecific taxa.
(PDF)
Statistics on the recovery of matK from samples obtained from silica-gel dried leaf material.
(PDF)
Ability of the supplementary plastid DNA barcode loci psbA–trnH, psbK–I and atpF–atpH to discriminate species and infraspecific taxa of Poa and Puccinellia.
(PDF)
Species from the Canadian Arctic Archipelago for which barcode data were. not obtained in the current study.
(PDF)
Frequency histogram showing the distribution of the number of individuals sampled per species. Putative hybrids are not included.
(PDF)
Numbers of rbcL and matK sequences recovered from each plant family. (A) Families with less than 50 samples recovered per family. (B) Families with more than 50 samples recovered per family.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Amaranthaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Apiaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Asteraceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Betulaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analysis of uncorrected p-distances of matK sequence data for Boraginaceae.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Brassicaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Campanulaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Caprifoliaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Caryophyllaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Celastraceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Cyperaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Diapensiaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analysis of uncorrected p-distances of rbcL sequence data for Dryopteridaceae.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Elaeagnaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analysis of uncorrected p-distances of rbcL sequence data for Equisetaceae.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Ericaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Fabaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Gentianaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analysis of uncorrected p-distances of rbcL sequence data for Haloragaceae.
(PDF)
Neighbour joining analysis of uncorrected p-distances of rbcL sequence data for Juncaceae.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Juncaginaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Lentibulariaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL sequence data for Linaceae.
(PDF)
Neighbour joining analysis of uncorrected p-distances of rbcL sequence data for Lycopodiaceae.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Melanthiaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Menyanthaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Montiaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Onagraceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Orchidaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Orobanchaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Papaveraceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analysis of uncorrected p-distances of rbcL sequence data for Pinales (Pinaceae, Cupressaceae).
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Plantaginaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Plumbaginaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Poaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Polemoniaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Polygonaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Potamogetonaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Primulaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Ranunculaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Rosaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Salicaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Saxifragaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Tofieldiaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Typhaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Puccinellia (Poaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of psbA–trnH sequence data for Puccinellia and Poa (Poaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of atpF–atpH sequence data for Puccinellia and Poa (Poaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of psbK–psbI sequence data for Puccinellia and Poa (Poaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Festuca (Poaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Poa (Poaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Pedicularis (Orobanchaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Salix (Salicaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Draba (Brassicaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Saxifraga (Saxifragaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Ranunculus (Ranunculaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Potentilla (Rosaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Carex (Cyperaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of matK sequence data for Carex and Kobresia (Cyperaceae). The tree combines data from the current study and previously published data from Le Clerc-Blain et al. [108], the latter prefaced with the project code "CAREX". .
(PDF)
Acknowledgments
We are grateful to Maria Kuzmina and staff at the Biodiversity Institute of Ontario, University of Guelph, for generating the barcode data, and Paul Hebert for his support of this work. We thank the many individuals who helped sample herbarium specimens, prepared plates for DNA extraction and sequencing, and scanned herbarium specimens, including Anna Ginter, Shan Leung, Tasfia Iqbal, and Cassandra Robillard. We thank Jennifer Doubt and Micheline Bouchard for their help in the National Herbarium of Canada (CAN) and for graciously accommodating urgent requests to mount barcode voucher specimens. This work is part of Working Group 1.10 (Polar Life) (http://ibol.org/wg-1-10-polar-life/, accessed 25 April 2011) of the International Barcode of Life Project (iBOL) (http://ibol.org/, accessed 25 April 2011).
Funding Statement
Laboratory work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Government of Canada through Genome Canada and the Ontario Genomics Institute (2008-0GI-ICI-03) in support of the International Barcode of Life Project, and the Canadian Museum of Nature. Fieldwork in the Arctic was supported by the Polar Continental Shelf Program (Natural Resources Canada), the Canadian Museum of Nature, and Parks Canada. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hudson JMG, Henry GHR (2009) Increased plant biomass in a High Arctic heath community from 1981 to 2008. Ecology 90: 2657–2663. doi: 10.1890/09-0102.1. PubMed: 19886474. [DOI] [PubMed] [Google Scholar]
- 2. Hudson JMG, Henry GHR, Cornwell WK (2011) Taller and larger: shifts in Arctic tundra leaf traits after 16 years of experimental warming. Glob Change Biol 17: 1013–1021. doi: 10.1111/j.1365-2486.2010.02294.x. [DOI] [Google Scholar]
- 3. Jia GJ, Epstein HE, Walker DA (2009) Vegetation greening in the Canadian arctic related to decadal warming. J Environ Monit 11: 2231–2238. doi: 10.1039/B911677J. PubMed: 20024021. [DOI] [PubMed] [Google Scholar]
- 4. Walker MD, Wahren CH, Hollister RD, Henry GHR, Ahlquist LE et al. (2006) Plant community responses to experimental warming across the tundra biome. Proc Natl Acad Sci U S A 103: 1342–1346. doi: 10.1073/pnas.0503198103. PubMed: 16428292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhatt US, Walker DA, Raynolds MK, Comiso JC, Epstein HE et al. (2010) Circumpolar Arctic tundra vegetation change is linked to sea-ice decline. Earth Interact 14: 1–20. doi: 10.1175/2010EI315.1. [DOI] [Google Scholar]
- 6. Pouliot D, Latifovic R, Olthof I (2009) Trends in vegetation NDVI from 1 km AVHRR data over Canada for the period 1985–2006. Int J Remote Sens 30: 149–168. doi: 10.1080/01431160802302090. [DOI] [Google Scholar]
- 7. Tape KEN, Sturm M, Racine C (2006) The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Glob Change Biol 12: 686–702. doi: 10.1111/j.1365-2486.2006.01128.x. [DOI] [Google Scholar]
- 8. Myers-Smith IH, Hik DS, Kennedy C, Cooley D, Johnstone JF et al. (2011) Expansion of canopy-forming willows over the twentieth century on Herschel Island, Yukon Territory, Canada. Ambio 40: 610–623. doi: 10.1007/s13280-011-0168-y. PubMed: 21954724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lantz TC, Gergel SE, Henry GHR (2010) Response of green alder (Alnus viridis subsp. fruticosa) patch dynamics and plant community composition to fire and regional temperature in north-western Canada. J Biogeogr 37: 1597–1610. doi: 10.1111/j.1365-2699.2010.02317.x. [DOI] [Google Scholar]
- 10. Forbes BC, Fauria MM, Zetterberg P (2010) Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Glob Change Biol 16: 1542–1554. doi: 10.1111/j.1365-2486.2009.02047.x. [DOI] [Google Scholar]
- 11. Stow DA, Hope A, McGuire D, Verbyla D, Gamon J et al. (2004) Remote sensing of vegetation and land-cover change in Arctic tundra ecosystems. Remote Sens Environ 89: 281–308. doi: 10.1016/j.rse.2003.10.018. [DOI] [Google Scholar]
- 12. Danby RK, Koh S, Hik DS, Price LW (2011) Four decades of plant community change in the alpine tundra of southwest Yukon, Canada. Ambio 40: 660–671. doi: 10.1007/s13280-011-0172-2. PubMed: 21954728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daniëls FJA, Molenaar JG (2011) Flora and vegetation of Tasiilaq, formerly Angmagssalik, Southeast Greenland: A comparison of data between around 1900 and 2007. Ambio 40: 650–659. doi: 10.1007/s13280-011-0171-3. PubMed: 21954727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daniëls FJA, de Molenaar JG, Chytrý M, Tichý L (2011) Vegetation change in Southeast Greenland? Tasiilaq revisited after 40 years. Appl Veg Sci 14: 230–241. doi: 10.1111/j.1654-109X.2010.01107.x. [DOI] [Google Scholar]
- 15. Callaghan TV, Christensen TR, Jantze EJ (2011) Plant and vegetation dynamics on Disko Island. Greenland: West: Snapshots separated by over 40 years. AMBIO 40: 624–637 doi: 10.1007/s13280-011-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harsch MA, Hulme PE, McGlone MS, Duncan RP (2009) Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol Lett 12: 1040–1049. doi: 10.1111/j.1461-0248.2009.01355.x. PubMed: 19682007. [DOI] [PubMed] [Google Scholar]
- 17. Arctic Circumpolar Vegetation Team (2003) Circumpolar Arctic Vegetation Map. (1:7,500,000 scale). Conservation of Arctic Flora and Fauna (CAFF) Map No. Anchorage, Alaska: U.S. Fish and Wildlife Service; p. 1. [Google Scholar]
- 18. Walker D, Raynolds M, Daniëls F, Einarsson E, Elvebakk A et al. (2005) The circumpolar Arctic vegetation map. J Veg Sci 16: 267–282. doi: 10.1111/j.1654-1103.2005.tb02365.x. [DOI] [Google Scholar]
- 19. Brown R (1823) Chloris Melvilliana: a list of plants collected in Melville Island (latitude 74-75 N. longitude 110-112 W.) in the year 1820; by the officers of the voyage of discovery under the orders of Captain Parry. With characters and descriptions of the new genera and species. London: William Clowes. 52 pp. doi: 10.5962/bhl.title.8114. [DOI] [Google Scholar]
- 20. Elven R, Murray DF, Razzhivin VY, Yurtsev BA (2011) Annotated checklist of the Panarctic Flora (PAF): Vascular plants version 1.0. Available: http://www.nhm2.uio.no/paf. Accessed continuously
- 21. Porsild AE (1957) Illustrated Flora of the Canadian Arctic Archipelago. Natl Museums Canada Bulletins 146, Biological Series 50: 1–209 [Google Scholar]
- 22. Porsild AE (1964) Illustrated Flora of the Canadian Arctic Archipelago, second Edition. National Museum of Canada Bulletin 146, Biological Series 50: 1–218 [Google Scholar]
- 23. Porsild AE, Cody WJ (1980) Vascular Plants of Continental Northwest Territories, Canada. Ottawa, Canada: National Museum of Natural Sciences, National Museums of Canada. 667 p [Google Scholar]
- 24. Hultén E (1968) Flora of Alaska and neighbouring territories: a manual of the vascular plants. Stanford, CA: Stanford University Press. 1032 pp. [Google Scholar]
- 25. Aiken SG, Dallwitz MJ, Consaul LL, McJannet CL, Boles RL et al. (2007) Flora of the Canadian Arctic Archipelago: Descriptions, Illustrations, Identification, and Information Retrieval [CD-ROM]. Ottawa: NRC Research Press, National Research Council of Canada; Available: http://nature.ca/en/research-collections/our-research/areas-expertise/botany/flora-canadian-arctic-archipelago. Accessed 6 December 2012. [Google Scholar]
- 26. Bennett BA, Catling PM, Cody WJ, Argus GW (2010. [2011]) New records of vascular plants in the Yukon Territory VIII. Can Field Nat 124: 1–27. [Google Scholar]
- 27. Cody WJ, Reading KL (2005) Additions and range extensions to the vascular plant flora of the continental Northwest Territories and Nunavut, Canada III. Can Field Nat 119: 276–290. [Google Scholar]
- 28. Schwarzenbach FH (2010) Botanical observations on the Penny Highlands of Baffin Island. Herstellung und Verlag: Books on Demand GmbH, Norderstedt. 159 p.
- 29. Saarela JM, Gillespie LJ, Consaul LL, Bull RD (2012) Balsam poplar (Populus balsamifera; Salicaceae) beyond the treeline in the western Canadian mainland Arctic (Northwest Territories). Arctic 65: 1–12. [Google Scholar]
- 30. Saarela JM, Gillespie LJ, Consaul LL, Bull RD (2013) Annotated checklist to the vascular plant flora of Tuktut Nogait National Park and the Melville Hills region (Canadian Low Arctic). Phytotaxa 102: 1–177. doi: 10.11646/phytotaxa.102.1.1. [DOI] [Google Scholar]
- 31. Hajibabaei M, Janzen DH, Burns JM, Hallwachs W, Hebert PDN (2006) DNA barcodes distinguish species of tropical Lepidoptera. Proc Natl Acad Sci U S A 103: 968–971. doi: 10.1073/pnas.0510466103. PubMed: 16418261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith MA, Rodriguez JJ, Whitfield JB, Deans AR, Janzen DH et al. (2008) Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. Proc Natl Acad Sci U S A 105: 12359–12364. doi: 10.1073/pnas.0805319105. PubMed: 18716001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kerr KCR, Stoeckle MY, Dove CJ, Weigt LA, Francis CM et al. (2007) Comprehensive DNA barcode coverage of North American birds. Mol Ecol Notes 7: 535–543. doi: 10.1111/j.1471-8286.2007.01670.x. PubMed: 18784793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fazekas AJ, Burgess KS, Kesanakurti PR, Graham SW, Newmaster SG et al. (2008) Multiple multilocus DNA barcodes from the plastid genome discriminate plant species equally well. PLOS ONE 3: e2802. doi: 10.1371/journal.pone.0002802. PubMed: 18665273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cho Y, Mower JP, Qiu YL, Palmer JD (2004) Mitochondrial substitution rates are extraordinarily elevated and variable in a genus of flowering plants. Proc Natl Acad Sci U S A 101: 17741–17746. doi: 10.1073/pnas.0408302101. PubMed: 15598738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mower JP, Touzet P, Gummow JS, Delph LF, Palmer JD (2007) Extensive variation in synonymous substitution rates in mitochondrial genes of seed plants. BMC Evol Biol 7: 135. doi: 10.1186/1471-2148-7-135. PubMed: 1768869610.1186%2F1471-2148-7-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kress WJ, Erickson DL (2007) A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLOS ONE 2: e508. doi: 10.1371/journal.pone.0000508. PubMed: 17551588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adams KL, Palmer JD (2003) Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol Phylogenet Evol 29: 380–395. doi: 10.1016/S1055-7903(03)00194-5. PubMed: 1461518110.1016%2FS1055-7903%2803%2900194-5 [DOI] [PubMed] [Google Scholar]
- 39. Chase MW, Salamin N, Wilkinson M, Dunwell JM, Kesanakurthi RP et al. (2005) Land plants and DNA barcodes: short-term and long-term goals. Philos Trans R Soc Lond B-Biol Sci 360: 1889–1895. doi: 10.1098/rstb.2005.1720. PubMed: 16214746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chase MW, Cowan RS, Hollingsworth PM, van den Berg C, Madrinan S et al. (2007) A proposal for a standardised protocol to barcode all land plants. Taxon 56: 295–299. [Google Scholar]
- 41. Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH (2005) Use of DNA barcodes to identify flowering plants. Proc Natl Acad Sci U S A 102: 8369–8374. doi: 10.1073/pnas.0503123102. PubMed: 15928076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Newmaster SG, Fazekas AJ, Ragupathy S (2006) DNA barcoding in land plants: evaluation of rbcL in a multigene tiered approach. Can J Bot 84: 335–341. doi: 10.1139/b06-047. [DOI] [Google Scholar]
- 43. Lahaye R, van der Bank M, Bogarin D, Warner J, Pupulin F et al. (2008) DNA barcoding the floras of biodiversity hotspots. Proc Natl Acad Sci U S A 105: 2923–2928. doi: 10.1073/pnas.0709936105. PubMed: 18258745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muellner AN, Schaefer H, Lahaye R (2011) Evaluation of candidate DNA barcoding loci for economically important timber species of the mahogany family (Meliaceae). Mol Ecol Resour 11: 450–460. doi: 10.1111/j.1755-0998.2011.02984.x. PubMed: 21481203. [DOI] [PubMed] [Google Scholar]
- 45. Ford CS, Ayres KL, Toomey N, Haider N, van Alphen Stahl J et al. (2009) Selection of candidate coding DNA barcoding regions for use on land plants. Bot J Linn Soc 159: 1–11. doi: 10.1111/j.1095-8339.2008.00938.x. [DOI] [Google Scholar]
- 46. Newmaster SG, Fazekas AJ, Steeves RAD, Janovec J (2008) Testing candidate plant barcode regions in the Myristicaceae. Mol Ecol Resour 8: 480–490. doi: 10.1111/j.1471-8286.2007.02002.x. PubMed: 21585825. [DOI] [PubMed] [Google Scholar]
- 47. Presting G (2006) Identification of conserved regions in the plastid genome: implications for DNA barcoding and biological function. Can J Bot 84: 1434. doi: 10.1139/b06-117. [DOI] [Google Scholar]
- 48. Hollingsworth ML, Clark A, Forrest LL, Richardson J, Pennington RT et al. (2009) Selecting barcoding loci for plants: evaluation of seven candidate loci with species-level sampling in three divergent groups of land plants. Mol Ecol Resour 9: 439–457. doi: 10.1111/j.1755-0998.2008.02439.x. PubMed: 21564673. [DOI] [PubMed] [Google Scholar]
- 49. Seberg O, Petersen G (2009) How many loci does it take to DNA barcode a crocus? PLOS ONE 4: e4598. doi: 10.1371/journal.pone.0004598. PubMed: 19240801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taberlet P, Coissac E, Pompanon F, Gielly L, Miquel C et al. (2007) Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. Nucleic Acids Res 35: 1–8. doi: 10.1093/nar/gkl1051. PubMed: 1692074410.1093%2Fnar%2Fgkl938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hollingsworth PM, Graham SW, Little DP (2011) Choosing and using a plant DNA barcode. PLOS ONE 6: e19254. doi: 10.1371/journal.pone.0019254. PubMed: 21637336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. CBOL Plant Working Group (2009) A DNA barcode for land plants. Proceedings of the National Academy of Sciences of the United States of America 106: 12794–12797. doi: 10.1073/pnas.0905845106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Executive Committee of the Consortium for the Barcode of Life (2009) Plant Working Group. Available: http://www.barcoding.si.edu/plant_working_group.html. Accessed 27 November 2012.
- 54. Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH (2005) Use of DNA barcodes to identify flowering plants. Proc Natl Acad Sci U S A 102: 8369–8374. doi: 10.1073/pnas.0503123102. PubMed: 15928076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hollingsworth PM (2011) Refining the DNA barcode for land plants. Proc Natl Acad Sci U S A 108: 19451–19452. doi: 10.1073/pnas.1116812108. PubMed: 22109553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yao H, Song J, Liu C, Luo K, Han J et al. (2010) Use of ITS2 region as the universal DNA barcode for plants and animals. PLOS ONE 5: e13102. doi: 10.1371/journal.pone.0013102. PubMed: 20957043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen S, Yao H, Han J, Liu C, Song J et al. (2010) Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLOS ONE 5: e8613. doi: 10.1371/journal.pone.0008613. PubMed: 20062805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Plant China BOL Group, Li D-Z, Gao L-M, Li H-T, Wang H et al. (2011) Comparative analysis of a large dataset indicates that internal transcribed spacer (ITS) should be incorporated into the core barcode for seed plants. Proc Natl Acad Sci U S A 108: 19641–19646. doi: 10.1073/pnas.1104551108. PubMed: 22100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xiang X-G, Hu HAO, Wang WEI, Jin X-H (2011) DNA barcoding of the recently evolved genus Holcoglossum (Orchidaceae: Aeridinae): a test of DNA barcode candidates. Mol Ecol Resour 11: 1012–1021. doi: 10.1111/j.1755-0998.2011.03044.x. PubMed: 21722327. [DOI] [PubMed] [Google Scholar]
- 60. Zhang C-Y, Wang F-Y, Yan H-F, Hao G, Hu C-M et al. (2012) Testing DNA barcoding in closely related groups of Lysimachia . p. L (Myrsinaceae: ). Molecular Ecology Resources 12: 98–108 doi: 10.1111/j.1755-0998.2011.03076.x. [DOI] [PubMed] [Google Scholar]
- 61. Ran J-H, Wang P-P, Zhao H-J, Wang X-Q (2010) A test of seven candidate barcode regions from the plastome in Picea (Pinaceae). J Integr Plant Biol 52: 1109–1126. doi: 10.1111/j.1744-7909.2010.00995.x. PubMed: 21106009. [DOI] [PubMed] [Google Scholar]
- 62. Yu W-B, Huang P-H, Ree RH, Liu M-L, Li D-Z et al. (2011) DNA barcoding of Pedicularis. p. L. (Orobanchaceae): Evaluating four universal barcode loci in a large and hemiparasitic genus. Journal of Systematics and Evolution 49: 425–437. doi: 10.1111/j.1759-6831.2011.00154.x. [DOI] [Google Scholar]
- 63. Arca M, Hinsinger DD, Cruaud C, Tillier A, Bousquet J et al. (2012) Deciduous trees and the application of universal DNA barcodes: A case study on the circumpolar Fraxinus . PLOS ONE 7: e34089. doi: 10.1371/journal.pone.0034089. PubMed: 22479532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li HQ, Chen JY, Wang S, Xiong SZ (2012) Evaluation of six candidate DNA barcoding loci in Ficus (Moraceae) of China. Mol Ecol Resour 12: 783–790. doi: 10.1111/j.1755-0998.2012.03147.x. PubMed: 22537273. [DOI] [PubMed] [Google Scholar]
- 65. Liu Y, Zhang L, Liu Z, Luo K, Chen S et al. (2012) Species identification of Rhododendron (Ericaceae) using the chloroplast deoxyribonucleic acid psbA-trnH genetic marker. Pharmacognosy Mag 8: 29–36. doi: 10.4103/0973-1296.93311. PubMed: 22438660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang J-B, Wang Y-P, Möller M, Gao L-M, Wu D (2012) Applying plant DNA barcodes to identify species of Parnassia (Parnassiaceae). Mol Ecol Resour 12: 267–275. doi: 10.1111/j.1755-0998.2011.03095.x. PubMed: 22136257. [DOI] [PubMed] [Google Scholar]
- 67. Yang H-Q, Dong Y-R, Gu Z-J, Liang N, Yang J-B (2012) A preliminary assessment of matK rbcL and trnH—psbA as DNA barcodes for Calamus (Arecaceae) species in China with a note on ITS. Annales Botanici Fennici 49: 319–330. 10.5735/085.049.0603 [DOI] [Google Scholar]
- 68. Piredda R, Simeone MC, Attimonelli M, Bellarosa R, Schirone B (2011) Prospects of barcoding the Italian wild dendroflora: oaks reveal severe limitations to tracking species identity. Mol Ecol Resour 11: 72–83. doi: 10.1111/j.1755-0998.2010.02900.x. PubMed: 21429102. [DOI] [PubMed] [Google Scholar]
- 69. Steven GN, Subramanyam R (2009) Testing plant barcoding in a sister species complex of pantropical Acacia (Mimosoideae, Fabaceae). Mol Ecol Resour 9: 172–180. doi: 10.1111/j.1755-0998.2009.02642.x. PubMed: 21564976. [DOI] [PubMed] [Google Scholar]
- 70. Pettengill JB, Neel MC (2010) An evaluation of candidate plant DNA barcodes and assignment methods in diagnosing 29 species in the genus Agalinis (Orobanchaceae). Am J Bot 97: 1391–1406. doi: 10.3732/ajb.0900176. PubMed: 21616891. [DOI] [PubMed] [Google Scholar]
- 71. Ren BQ, Xiang XG, Chen ZD (2010) Species identification of Alnus (Betulaceae) using nrDNA and cpDNA genetic markers. Mol Ecol Resour 10: 594–605. doi: 10.1111/j.1755-0998.2009.02815.x. PubMed: 21565064. [DOI] [PubMed] [Google Scholar]
- 72. Roy S, Tyagi A, Shukla V, Kumar A, Singh UM et al. (2010) Universal plant DNA barcode loci may not work in complex groups: A case study with Indian Berberis species. PLOS ONE 5: e13674. doi: 10.1371/journal.pone.0013674. PubMed: 21060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yan H-F, Hao G, Hu C-M, Ge X-J (2011) DNA barcoding in closely related species: A case study of Primula L. sect. Proliferae Pax (Primulaceae) in China. J Syst Evolution 49: 225–236. doi: 10.1111/j.1759-6831.2011.00115.x. [DOI] [Google Scholar]
- 74. Shi L-C, Zhang J, Han J-P, Song J-Y, Yao H et al. (2011) Testing the potential of proposed DNA barcodes for species identification of Zingiberaceae. J Syst Evolution 49: 261–266. doi: 10.1111/j.1759-6831.2011.00133.x. [DOI] [Google Scholar]
- 75. Cai Z-M, Zhang Y-X, Zhang L-N, Gao L-M, Li D-Z (2012) Testing four candidate barcoding markers in temperate woody bamboos (Poaceae: Bambusoideae). J Syst Evolution 50: 527–539. doi: 10.1111/j.1759-6831.2012.00216.x. [DOI] [Google Scholar]
- 76. Du Z-Y, Qimike A, Yang C-F, Chen J-M, Wang Q-F (2011) Testing four barcoding markers for species identification of Potamogetonaceae. J Syst Evolution 49: 246–251. doi: 10.1111/j.1759-6831.2011.00131.x. [DOI] [Google Scholar]
- 77. Gao T, Yao H, Song J, Zhu Y, Liu C et al. (2010) Evaluating the feasibility of using candidate DNA barcodes in discriminating species of the large Asteraceae family. BMC Evol Biol 10: 1–7. doi: 10.1186/1471-2148-10-324. PubMed: 20044934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yesson C, Bárcenas RT, Hernández HM, De La Luz Ruiz-Maqueda M, Prado A et al. (2011) DNA barcodes for Mexican Cactaceae, plants under pressure from wild collecting. Mol Ecol Resour 11: 775–783. doi: 10.1111/j.1755-0998.2011.03009.x. PubMed: 21457479. [DOI] [PubMed] [Google Scholar]
- 79. Pang X, Song J, Zhu Y, Xu H, Huang L et al. (2011) Applying plant DNA barcodes for Rosaceae species identification. Cladistics 27: 165-170. doi: 10.1111/j.1096-0031.2010.00328.x. [DOI] [PubMed] [Google Scholar]
- 80. Luo K, Chen S, Chen K, Song J, Yao H et al. (2010) Assessment of candidate plant DNA barcodes using the Rutaceae family. Sci China Life Sci 53: 701–708. doi: 10.1007/s11427-010-4009-1. PubMed: 20602273. [DOI] [PubMed] [Google Scholar]
- 81. Drumwright AM, Allen BW, Huff KA, Ritchey PA, Cahoon AB (2011) Survey and DNA barcoding of Poaceae in Flat Rock Cedar Glades and Barrens State Natural Area, Murfreesboro, Tennessee. Castanea 76: 300–310. doi: 10.2179/11-005.1. [DOI] [Google Scholar]
- 82. Maia VH, Mata CS Sd; Franco LO, Cardoso MA, Cardoso SRS et al. (2012) DNA barcoding Bromeliaceae: achievements and pitfalls. PLOS ONE 7: e29877. doi: 10.1371/journal.pone.0029877. PubMed: 22253812. 10.1371/journal.pone.0029877 PubMed: 22253812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kelly LJ, Ameka GK, Chase MW (2010) DNA barcoding of African Podostemaceae (river-weeds): A test of proposed barcode regions. Taxon 59: 251–260. [Google Scholar]
- 84. Liu Y, Yan H-F, Cao T, Ge X-J (2010) Evaluation of 10 plant barcodes in Bryophyta (Mosses). J Syst Evolution 48: 36–46. doi: 10.1111/j.1759-6831.2009.00063.x. [DOI] [Google Scholar]
- 85. Armenise L, Simeone M, Piredda R, Schirone B (2012) Validation of DNA barcoding as an efficient tool for taxon identification and detection of species diversity in Italian conifers. Eur J Forest Res 131: 1337–1353. doi: 10.1007/s10342-012-0602-0. [DOI] [Google Scholar]
- 86. Lucas C, Thangaradjou T, Papenbrock J (2012) Development of a DNA barcoding system for seagrasses: Successful but not simple. PLOS ONE 7: e29987. doi: 10.1371/journal.pone.0029987. PubMed: 22253849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. de Groot GA, During HJ, Maas JW, Schneider H, Vogel JC et al. (2011) Use of rbcL and trnL-F as a two-locus DNA barcode for identification of NW-European ferns: an ecological perspective. PLOS ONE 6: e16371. doi: 10.1371/journal.pone.0016371. PubMed: 21298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li M, Cao H, But PP-H, Shaw P-C (2011) Identification of herbal medicinal materials using DNA barcodes. J Syst Evolution 49: 271–283. doi: 10.1111/j.1759-6831.2011.00132.x. [DOI] [Google Scholar]
- 89. Kool A, de Boer HJ, Krüger Å, Rydberg A, Abbad A et al. (2012) Molecular identification of commercialized medicinal plants in southern Morocco. PLOS ONE 7: e39459. doi: 10.1371/journal.pone.0039459. PubMed: 22761800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Madesis P, Ganopoulos I, Ralli P, Tsaftaris A (2012) Barcoding the major Mediterranean leguminous crops by combining universal chloroplast and nuclear DNA sequence targets. Genet Mol Res 11: 2548–2558. doi: 10.4238/2012.July.10.10. PubMed: 22869075. [DOI] [PubMed] [Google Scholar]
- 91. Nicolè S, Erickson DL, Ambrosi D, Bellucci E, Lucchin M et al. (2011) Biodiversity studies in Phaseolus species by DNA barcoding. Genome 54: 529–545. doi: 10.1139/g11-018. PubMed: 21777058. [DOI] [PubMed] [Google Scholar]
- 92. Ghahramanzadeh R, Esselink G, Kodde LP, Duistermaat H, van Valkenburg JLCH et al. (2012) Efficient distinction of invasive aquatic plant species from non-invasive related species using DNA barcoding. Molecular Ecology Resources: n/a-n/a. doi: 10.1111/1755-0998.12020. [DOI] [PubMed]
- 93. Van De Wiel CCM, Van Der Schoot J, Van Valkenburg JLCH, Duistermaat H, Smulders MJM (2009) DNA barcoding discriminates the noxious invasive plant species, floating pennywort (Hydrocotyle ranunculoides L.f.), from non-invasive relatives. Mol Ecol Resour 9: 1086–1091. doi: 10.1111/j.1755-0998.2009.02547.x. PubMed: 21564846. [DOI] [PubMed] [Google Scholar]
- 94. Kress WJ, Erickson DL, Jones FA, Swenson NG, Perez R et al. (2009) Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proc Natl Acad Sci U S A 106: 18621–18626. doi: 10.1073/pnas.0909820106. PubMed: 19841276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Burgess KS, Fazekas AJ, Kesanakurti PR, Graham SW, Husband BC et al. (2011) Discriminating plant species in a local temperate flora using the rbcL+ matK DNA barcode. Methods Ecol Evolution 2: 333–340. doi: 10.1111/j.2041-210X.2011.00092.x. [DOI] [Google Scholar]
- 96. Ebihara A, Nitta JH, Ito M (2010) Molecular species identification with rich floristic sampling: DNA barcoding the pteridophyte flora of Japan. PLOS ONE 5: e15136. doi: 10.1371/journal.pone.0015136. PubMed: 21170336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gonzalez MA, Baraloto C, Engel J, Mori SA, Pétronelli P et al. (2009) Identification of Amazonian trees with DNA barcodes. PLOS ONE 4: e7483. doi: 10.1371/journal.pone.0007483. PubMed: 19834612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. de Vere N, Rich TCG, Ford CR, Trinder SA, Long C et al. (2012) DNA barcoding the native flowering plants and conifers of Wales. PLOS ONE 7: e37945. doi: 10.1371/journal.pone.0037945. PubMed: 22701588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kesanakurti PR, Fazekas AJ, Burgess KS, Percy DM, Newmaster SG et al. (2011) Spatial patterns of plant diversity below ground as revealed by DNA barcoding. Mol Ecol 20: 1289–1302. doi: 10.1111/j.1365-294X.2010.04989.x. PubMed: 21255172. [DOI] [PubMed] [Google Scholar]
- 100. Pryer KM, Schuettpelz E, Huiet L, Grusz AI, Rothfels CJ et al. (2010) DNA barcoding exposes a case of mistaken identity in the fern horticultural trade. Mol Ecol Resour 10: 979–985. doi: 10.1111/j.1755-0998.2010.02858.x. PubMed: 21565107. [DOI] [PubMed] [Google Scholar]
- 101. Wallace LJ, Boilard SMAL, Eagle SHC, Spall JL, Shokralla S et al. (2012) DNA barcodes for everyday life: Routine authentication of Natural Health Products. Food Res Int 49: 446–452. doi: 10.1016/j.foodres.2012.07.048. [DOI] [Google Scholar]
- 102. Valentini A, Miquel C, Nawaz MA, Bellemain E, Coissac E et al. (2009) New perspectives in diet analysis based on DNA barcoding and parallel pyrosequencing: the trnL approach. Mol Ecol Resour 9: 51–60. doi: 10.1111/j.1755-0998.2008.02352.x. PubMed: 21564566. [DOI] [PubMed] [Google Scholar]
- 103. Jurado-Rivera JA, Vogler AP, Reid CAM, Petitpierre E, Gómez-Zurita J (2009) DNA barcoding insect–host plant associations. Proc R Soc Lond B Biol Sci 276: 639–648. doi: 10.1098/rspb.2008.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bell D, Long DG, Forrest AD, Hollingsworth ML, Blom HH et al. (2012) DNA barcoding of European Herbertus (Marchantiopsida, Herbertaceae) and the discovery and description of a new species. Mol Ecol Resour 12: 36–47. doi: 10.1111/j.1755-0998.2011.03053.x. PubMed: 21824334. [DOI] [PubMed] [Google Scholar]
- 105. Milstein D, Saunders GW (2012) DNA barcoding of Canadian Ahnfeltiales (Rhodophyta) reveals a new species – Ahnfeltia borealis sp. nov. Phycologia 51: 247–259. doi: 10.2216/11-40.1. [DOI] [Google Scholar]
- 106. Soininen EM, Valentini A, Coissac E, Miquel C, Gielly L et al. (2009) Analysing diet of small herbivores: the efficiency of DNA barcoding coupled with high-throughput pyrosequencing for deciphering the composition of complex plant mixtures. Front Zool 6: 16. doi: 10.1186/1742-9994-6-16. PubMed: 19695081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sønstebø JH, Gielly L, Brysting AK, Elven R, Edwards M et al. (2010) Using next-generation sequencing for molecular reconstruction of past Arctic vegetation and climate. Mol Ecol Resour 10: 1009–1018. doi: 10.1111/j.1755-0998.2010.02855.x. PubMed: 21565110. [DOI] [PubMed] [Google Scholar]
- 108. Le Clerc-Blain J, Starr JR, Bull RD, Saarela JM (2010) A regional approach to plant DNA barcoding provides high species resolution of sedges (Carex and Kobresia, Cyperaceae) in the Canadian Arctic Archipelago. Mol Ecol Resour 10: 69–91. doi: 10.1111/j.1755-0998.2009.02725.x. PubMed: 21564992. [DOI] [PubMed] [Google Scholar]
- 109. Kuzmina ML, Johnson KL, Barron HR, Hebert PDN (2012) Identification of the vascular plants of Churchill, Manitoba, using a DNA barcode library. BMC Ecol 12: 25. doi: 10.1186/1472-6785-12-25. PubMed: 23190419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ratnasingham S, Hebert PDN; (2007) BOLD: The Barcode of Life Data System. Retrieved onpublished at whilst December year 1111 from http://www.barcodinglife.org. Molecular Ecology Notes 7: 355–364 doi:10.1111/j.1471-8286.2007.01678.x. [DOI] [PMC free article] [PubMed]
- 111. Christenhusz MJM, Zhang Z-C, Schneider H (2011) A linear sequence of extant families and genera of lycophytes and ferns. Phytotaxa 19: 7–54. [Google Scholar]
- 112. Christenhusz MJM, Reveal JL, Farjon A, Gardner MF, Mill RR et al. (2011) A new classification and linear sequence of extant gymnosperms. Phytotaxa 19: 55–70. [Google Scholar]
- 113. Smith A, Pryer K, Schuettpelz E, Korall P, Schneider H et al. (2006) A classification for extant ferns. Taxon 55: 705–731. doi: 10.2307/2506564610.2307 %2F25065646 [DOI] [Google Scholar]
- 114. Angiosperm Phylogeny Group III (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161: 105–121. doi: 10.1111/j.1095-8339.2009.00996.x. [DOI] [Google Scholar]
- 115. Cody WJ (2000) Flora of the Yukon Territory. second Edition. Ottawa: NRC Research Press. 669 pp. [Google Scholar]
- 116. Flora of North America Editorial Committee, editor (1993+) Flora of North America North of Mexico. 16+ volumes. New York and Oxford: Oxford University Press. [Google Scholar]
- 117. Aiken SG, Lefkovitch LP (1990) Arctagrostis (Poaceae, tribe Pooideae) in North America and Greenland. Can J Bot 68: 2422–2432. doi: 10.1139/b90-308. [DOI] [Google Scholar]
- 118. Harris JG (2006) Five new subspecies of Braya (Brassicaceae) from Canada. Novon 16: 344–353. doi:10.3417/1055-3177(2006)16[344:FNSOBB]2.0.CO;2. [Google Scholar]
- 119. Packer JG (1963) The taxonomy of some North American species of Chrysosplenium l., section Alternifolia Franchet. Can J Bot 41: 85–103. doi: 10.1139/b63-009. [DOI] [Google Scholar]
- 120. Elven R, Al-Shehbaz IA (2008) Draba simmonsii (Brassicaceae), a new species of the D. micropetala complex from the Canadian Arctic Archipelago. Novon 18: 325–329. doi: 10.3417/2007178. [DOI] [Google Scholar]
- 121. Cayouette J (2004) A taxonomic review of the Eriophorum russeolum--E. scheuchzeri complex (Cyperaceae) in North America. Sida 21: 791–814. [Google Scholar]
- 122. Aiken SG, Consaul LL, Lefkovitch LP (1995) Festuca endlundiae (Poaceae), a high arctic, new species compared enzymatically and morphologically with similar Festuca species. Syst Bot 20: 374–392. doi: 10.2307/2419501. [DOI] [Google Scholar]
- 123. Aiken SG, Consaul LL, Spidle A, May B (1994) Allozyme and morphological observations on Festuca hyperborea, compared with F. baffinensis and F. Brachyphylla (Poaceae) from the Canadian Arctic. Nord J Bot 14: 137–143. doi: 10.1111/j.1756-1051.1994.tb00580.x. [DOI] [Google Scholar]
- 124. Frederiksen S (1981) Festuca vivipara (Poaceae) in the North Atlantic area. Nord J Bot 1: 277–292. doi: 10.1111/j.1756-1051.1981.tb00697.x. [DOI] [Google Scholar]
- 125. Catling P, Spicer K (1987) The perennial Juncus of section Poiophylli in the Canadian prairie provinces. Can J Bot 65: 750–760. doi: 10.1139/b87-100. [DOI] [Google Scholar]
- 126. Kirschner J (2002) Juncaceae 1: Rostkovia to Luzula. Canberra, Australia: ABRS part 6.
- 127. Solstad H (2008) Taxonomy and evolution of the diploid and polyploid Papaver sect. Meconella (Papaveraceae). Philosophiae doctor dissertation, Faculty of Mathematics and Natural Sciences, National Centre for Biosystematics, Natural History Museum, Oslo: University of Oslo; . 237 p [Google Scholar]
- 128. Molau U, Murray DF (1996) Taxonomic revision of the Pedicularis sudetica complex (Scrophulariaceae): the Arctic species. Symbolae Bot Upsalienses 31: 33–46. [Google Scholar]
- 129. Cherniawsky DM, Bayer RJ (1998) Systematics of North American Petasites (Asteraceae: Senecioneae). I. Morphometric analyses. Can J Bot 76: 23–36. doi: 10.1139/b97-152. [DOI] [Google Scholar]
- 130. Cherniawsky DM, Bayer RJ (1998) Systematics of North American Petasites (Asteraceae: Senecioneae). III. A taxonomic revision. Can J Bot 76: 2061–2075. doi: 10.1139/cjb-76-12-2061Retrieved onpublished at whilst December year 1111 from . doi:10.1139/b98-222. [DOI] [Google Scholar]
- 131. Gillespie LJ, Boles R (2001) Phylogenetic relationships and infraspecific variation in Canadian Arctic Poa based on chloroplast DNA restriction site data. Can J Bot 79: 679–701. doi: 10.1139/b01-036. [DOI] [Google Scholar]
- 132. Gillespie LJ, Consaul LL, Aiken SG (1997) Hybridization and the origin of the arctic grass Poa hartzii (Poaceae): evidence from morphology and chloroplast DNA restriction site data. Can J Bot 75: 1978–1997. doi: 10.1139/b97-910. [DOI] [Google Scholar]
- 133. Soreng RJ (1991) Systematics of the "Epiles" group of Poa (Poaceae). Syst Bot 16: 507–528. doi: 10.2307/2419340. [DOI] [Google Scholar]
- 134. Consaul LL, Gillespie LJ (2001) A re-evaluation of species limits in Canadian Arctic Island Puccinellia (Poaceae): resolving key characters. Can J Bot 79: 927–956. doi: 10.1139/cjb-79-8-927. [DOI] [Google Scholar]
- 135. Consaul LL, Gillespie LJ, MacInnes KI (2005) Addition to the flora of Canada? A specimen from the Arctic Archipelago, Northwest Territories links two allopatric species of alkali grass, Puccinellia . Can Field Nat 119: 497–506. [Google Scholar]
- 136. Consaul LL, Gillespie LJ, Waterway MJ (2008) A new species of alkali grass (Puccinellia, Poaceae) from the western North American Arctic. Novon 18: 16–20. doi: 10.3417/2007029. [DOI] [Google Scholar]
- 137. Consaul LL, Gillespie LJ, Waterway MJ (2008) Systematics of North American Arctic diploid Puccinellia (Poaceae): morphology, DNA content, and AFLP markers. Syst Bot 33: 251–261. doi: 10.1600/036364408784571662. [DOI] [Google Scholar]
- 138. Consaul LL, Gillespie LJ, Waterway MJ (2008) Systematics of three North American polyploid arctic alkali grasses (Puccinellia, Poaceae): morphology, ploidy, and AFLP markers. Botany 86: 916–937. doi: 10.1139/B08-073. [DOI] [Google Scholar]
- 139. Consaul LL, Gillespie LJ, Waterway MJ (2010) Evolution and polyploid origins in North American Arctic Puccinellia (Poaceae) based on nuclear ribosomal spacer and chloroplast DNA sequences. Am J Bot 97: 324–336. doi: 10.3732/ajb.0900180. PubMed: 21622393. [DOI] [PubMed] [Google Scholar]
- 140. Consaul LL, Gillespie LJ, Waterway MJ (2010) Polyploid speciation and evolution in Arctic Puccinellia (Poaceae: Puccinellinae) - a review. In: Seberg O, Petersen G, Barfod AS, Davis JI. Diversity, phylogeny, and evolution in the monocotyledons. Aarhus: Aarhus University Press; pp. 645–662. [Google Scholar]
- 141. Sørensen TJ (1953) A revision of the Greenland species of Puccinellia Parl. with contribution to our knowledge of the arctic Puccinellia flora in general. Meddelelser Om Grønland 136: 1–179. [Google Scholar]
- 142. Swallen JR (1944) The Alaskan species of Puccinellia . Journal of The Washington Academy of Sciences; 34: 16–24 [Google Scholar]
- 143. Fernald ML, Weatherby CA (1916) The genus Puccinellia in eastern North America. Rhodora 18: 1–23. [Google Scholar]
- 144. Brouillet L, Desmet P, Coursol F, Meades SJ, Favreau M et al. (2010+) Database of Vascular Plants of Canada (VASCAN). Available: http://data.canadensys.net/vascan/. Retrieved onpublished at whilst December year 1111 from 10.5886/1bft7W5f [DOI]
- 145. Desmet P, Brouillet L (2013) Database of Vascular Plants of Canada (VASCAN): a community contributed taxonomic checklist of all vascular plants of Canada, Saint Pierre and Miquelon, and Greenland. Phytokeys 25: 55–67. doi: 10.3897/phytokeys.25.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tropicos Botanical information system at the Missouri Botanical Garden. Available: www.tropicos.org. Accessed continuously
- 147. The International Plant Names Index (2012). Available: http://www.ipni.org. Accessed continuously
- 148. Ivanova N, Kuzmina M, Fazekas A (2011) Canadian Center for DNA Barcoding ( CCDB) Protocols. Glass fiber plate DNA extraction protocol for plants, fungi, echinoderms and mollusks: Manual protocol employing centrifugation. Available: http://www.dnabarcoding.ca/CCDB_DOCS/CCDB_DNA_Extraction-Plants.pdf.
- 149. Ivanova N, Fazekas A, Hebert P (2008) Semi-automated, membrane-based protocol for DNA isolation from plants. Plant Mol Biol Rep 26: 186–198. doi: 10.1007/s11105-008-0029-4. [DOI] [Google Scholar]
- 150. Fazekas AJ, Kuzmina ML, Newmaster SG, Hollingsworth PM (2012) DNA barcoding methods for land plants. In: Kress WJ, Erickson DL. Methods in Molecular Biology. Humana Press; pp. 223–252. [DOI] [PubMed] [Google Scholar]
- 151. Kuzmina M, Ivanova N (2011) Canadian Center for DNA Barcoding ( CCDB) Protocols. PCR amplification for plants and fungi. Available: http://www.dnabarcoding.ca/CCDB_DOCS/CCDB_Amplification-Plants.pdf.
- 152. Ivanova N, Grainger C (2006) Pre-made frozen PCR and sequencing plates. Canadian Center for DNA Barcoding Advances: Methods Release No. 4. Available: http://www.dnabarcoding.ca/CCDB_DOCS/CCDB_Advances_Methods_Release_No4_Dec1st_2006.pdf.
- 153. Ivanova N, Grainger C (2005) Canadian Center for DNA Barcoding ( CCDB) Protocols. Sequencing. Available: http://www.dnabarcoding.ca/CCDB_DOCS/CCDB_Sequencing.pdf.
- 154. Levin RA, Wagner WL, Hoch PC, Nepokroeff M, Pires JC et al. (2003) Family-level relationships of Onagraceae based on chloroplast rbcL and ndhF data. Am J Bot 90: 107–115. doi: 10.3732/ajb.90.1.107. PubMed: 21659085. [DOI] [PubMed] [Google Scholar]
- 155. Soltis PS, Soltis DE, Smiley CJ (1992) An rbcL sequence from a Miocene Taxodium (bald cypress). Proc Natl Acad Sci U S A 89: 449–451. doi: 10.1073/pnas.89.1.449. PubMed: 1729716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Fofana B, Harvengt L, Baudoin J, Du Jardin P (1997) New primers for the polymerase chain amplification of cpDNA intergenic spacers in Phaseolus phylogeny. Belg J Bot 129: 118–122. [Google Scholar]
- 157. Kuzmina M, Ivanova N (2011) Canadian Center for DNA Barcoding ( CCDB) Protocols. Primers sets for plants and fungi. Available: http://www.dnabarcoding.ca/CCDB_DOCS/CCDB_PrimerSets-Plants.pdf.
- 158. Cuénoud P, Savolainen V, Chatrou LW, Powell M, Grayer RJ et al. (2002) Molecular phylogenetics of Caryophyllales based on nuclear 18S rDNA and plastid rbcL, atpB, and matK DNA sequences. Am J Bot 89: 132–144. doi: 10.3732/ajb.89.1.132. PubMed: 21669721. [DOI] [PubMed] [Google Scholar]
- 159. Tate JA, Simpson BB (2003) Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Syst Bot 28: 723–737. [Google Scholar]
- 160. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. doi: 10.1093/nar/gkh340. PubMed: 15034147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Meier R, Shiyang K, Vaidya G, Ng PKL (2006) DNA barcoding and taxonomy in Diptera: A tale of high intraspecific variability and low identification success. Syst Biol 55: 715–728. doi: 10.1080/10635150600969864. PubMed: 17060194. [DOI] [PubMed] [Google Scholar]
- 162. Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol Electron 4: 1–9. [Google Scholar]
- 163. Srivathsan A, Meier R (2011) On the inappropriate use of Kimura 2 parameter (K2P) divergences in the DNA barcoding literature. Cladistics 28: 190–194. doi: 10.1111/j.1096-0031.2011.00370.x. [DOI] [PubMed] [Google Scholar]
- 164. Collins RA, Boykin LM, Cruickshank RH, Armstrong KF (2012) Barcoding's next top model: an evaluation of nucleotide substitution models for specimen identification. Methods Ecol Evolution 3: 457–465. doi: 10.1111/j.2041-210X.2011.00176.x. [DOI] [Google Scholar]
- 165. Jeanson ML, Labat JN, Little DP (2011) DNA barcoding: a new tool for palm taxonomists? Ann Bot 108: 1445–1451. doi: 10.1093/aob/mcr158. PubMed: 21757475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Pang X, Luo H, Sun C (2012) Assessing the potential of candidate DNA barcodes for identifying non-flowering seed plants. Plant Biol 14: 839–844. doi: 10.1111/j.1438-8677.2011.00554.x. PubMed: 22309105. [DOI] [PubMed] [Google Scholar]
- 167. Li Y, Gao L-M, Poudel RC, Li D-Z, Forrest A (2011) High universality of matK primers for barcoding gymnosperms. J Syst Evolution 49: 169–175. doi: 10.1111/j.1759-6831.2011.00128.x. [DOI] [Google Scholar]
- 168. Sass C, Little DP, Stevenson DW, Specht CD (2007) DNA barcoding in the Cycadales: Testing the potential of proposed barcoding markers for species identification of cycads. PLOS ONE 2: e1154. doi: 10.1371/journal.pone.0001154. PubMed: 17987130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Liu JIE, MÖLler M, Gao L-M, Zhang D-Q, Li D-Z (2011) DNA barcoding for the discrimination of Eurasian yews (Taxus L., Taxaceae) and the discovery of cryptic species. Mol Ecol Resour 11: 89–100. doi: 10.1111/j.1755-0998.2010.02907.x. PubMed: 21429104. [DOI] [PubMed] [Google Scholar]
- 170. Duffy AM, Kelchner SA, Wolf PG (2009) Conservation of selection on matK following an ancient loss of its flanking intron. Gene 438: 17–25. doi: 10.1016/j.gene.2009.02.006. PubMed: 19236909. [DOI] [PubMed] [Google Scholar]
- 171. Wolf PG, Roper JM, Duffy AM (2010) The evolution of chloroplast genome structure in ferns. Genome 53: 731–738. doi: 10.1139/G10-061. PubMed: 20924422. [DOI] [PubMed] [Google Scholar]
- 172. Kuo LY, Li FW, Chiou WL, Wang CN (2011) First insights into fern matK phylogeny. Mol Phylogenet Evol 59: 556–566. doi: 10.1016/j.ympev.2011.03.010. PubMed: 21402161. [DOI] [PubMed] [Google Scholar]
- 173. Li FW, Kuo LY, Rothfels CJ, Ebihara A, Chiou WL et al. (2011) RbcL and matK earn two thumbs up as the core DNA barcode for ferns. PLoS ONE 6: e26597. doi: 10.1371/journal.pone.0026597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Schaefer H, Hardy OJ, Silva L, Barraclough TG, Savolainen V (2011) Testing Darwin’s naturalization hypothesis in the Azores. Ecol Lett 14: 389–396. doi: 10.1111/j.1461-0248.2011.01600.x. PubMed: 21320262. [DOI] [PubMed] [Google Scholar]
- 175. Starr JR, Naczi RFC, Chouinard BN (2009) Plant DNA barcodes and species resolution in sedges (Carex, Cyperaceae). Mol Ecol Resour 9: 151–163. doi: 10.1111/j.1755-0998.2009.02640.x. PubMed: 21564974. [DOI] [PubMed] [Google Scholar]
- 176. Dunning LT, Savolainen V (2010) Broad-scale amplification of matK for DNA barcoding plants, a technical note. Bot J Linn Soc 164: 1–9. doi: 10.1111/j.1095-8339.2010.01071.x. [DOI] [Google Scholar]
- 177. Johnson LA, Soltis DE (1994) matK DNA sequences and phylogenetic reconstruction in Saxifragaceae s. str. Syst Bot 19: 143–156. doi: 10.2307/241971810.2307 %2F2419718 [DOI] [Google Scholar]
- 178. Johnson LA, Soltis DE (1995) Phylogenetic inference in Saxifragaceae sensu stricto and Gilia (Polemoniaceae) using matK sequences. Ann Mo Bot Gard 82: 149–175. doi: 10.2307/239987510.2307 %2F2419718 [DOI] [Google Scholar]
- 179. Soltis DE, Kuzoff RK, Conti E, Gornall R, Ferguson K (1996) matK and rbcL gene sequence data indicate that Saxifraga (Saxifragaceae) is polyphyletic. Am J Bot: 371–382 10.2307%2F2446171 [Google Scholar]
- 180. Sarkar IN, Planet PJ, Desalle ROB (2008) CAOS software for use in character-based DNA barcoding. Mol Ecol Resour 8: 1256–1259. doi: 10.1111/j.1755-0998.2008.02235.x. PubMed: 21586014. [DOI] [PubMed] [Google Scholar]
- 181. Little DP, Stevenson DW (2007) A comparison of algorithms for the identification of specimens using DNA barcodes: examples from gymnosperms. Cladistics 23: 1–21. doi: 10.1111/j.1096-0031.2006.00126.x. [DOI] [PubMed] [Google Scholar]
- 182. Little DP (2011) DNA barcode sequence identification incorporating taxonomic hierarchy and within taxon variability. PLOS ONE 6: e20552. doi: 10.1371/journal.pone.0020552. PubMed: 21857897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Austerlitz F, David O, Schaeffer B, Bleakley K, Olteanu M et al. (2009) DNA barcode analysis: a comparison of phylogenetic and statistical classification methods. BMC Bioinformatics 10: S10. doi: 10.1186/1471-2105-10-S14-S10. PubMed: 19900297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Brysting AK, Fay MF, Leitch IJ, Aiken SG (2004) One or more species in the arctic grass genus Dupontia? - a contribution to the Panarctic Flora project. Taxon 53: 365–382. doi: 10.2307/413561510.2307 %2F4135615 [DOI] [Google Scholar]
- 185. Brysting AK, Aiken SG, Lefkovitch LP, Boles RL; (2003) Dupontia (Poaceae) in North America. Can J Bot 81: 769–779. doi: 10.1139/b03-067769-779, [DOI] [Google Scholar]
- 186. Gillespie LJ, Soreng RJ, Bull RD, Jacobs SWL, Refulio-Rodriguez NF (2008) Phylogenetic relationships in subtribe Poinae (Poaceae, Poeae) based on nuclear ITS and plastid trnT-trnL-trnF sequences. Botbot 86: 938–967. doi: 10.1139/b08-076. [DOI] [Google Scholar]
- 187. Gillespie L, Archambault A, Soreng R (2007) Phylogeny of Poa (Poaceae) based on trnT-trnF sequence data: major clades and basal relationships. Aliso 23: 420–434. [Google Scholar]
- 188. Quintanar A, Castroviejo S, Catalán P (2007) Phylogeny of the tribe Aveneae (Pooideae, Poaceae) inferred from plastid trnT-F and nuclear ITS sequences. Am J Bot 94: 1554–1569. doi: 10.3732/ajb.94.9.1554. PubMed: 21636521. [DOI] [PubMed] [Google Scholar]
- 189. Saarela JM, Liu Q, Peterson PM, Soreng RJ, Paszko B (2010) Phylogenetics of the grass 'Aveneae-type plastid DNA clade' (Poaceae: Pooideae, Poeae) based on plastid and nuclear ribosomal DNA sequence data. In: Seberg O, Petersen G, Barfod A, Davis JI. Diversity, phylogeny, and evolution in the monocotyledons. Denmark: Aarhus University Press; pp. 557–587. [Google Scholar]
- 190. Pang X, Song J, Zhu Y, Xu H, Huang L et al. (2011) Applying plant DNA barcodes for Rosaceae species identification. Cladistics 27: 165–170. doi: 10.1111/j.1096-0031.2010.00328.x. [DOI] [PubMed] [Google Scholar]
- 191. Alice LA, Campbell CS (1999) Phylogeny of Rubus (Rosaceae) based on nuclear ribosomal DNA internal transcribed spacer region sequences. Am J Bot 86: 81–97. doi: 10.2307/2656957. PubMed: 2168034810.2307%2F2656957 [DOI] [PubMed] [Google Scholar]
- 192. Gao T, Yao H, Song J, Zhu Y, Liu C et al. (2010) Evaluating the feasibility of using candidate DNA barcodes in discriminating species of the large Asteraceae family. BMC Evol Biol 10: 324. doi: 10.1186/1471-2148-10-324. PubMed: 20977734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Tkach NV, Hoffmann MH, Röser M, Korobkov AA, Von Hagen KB; (2008) Parallel evolutionary patterns in multiple lineages of Arctic Artemisia L (Asteraceae: ). Evolution 62: 184–198 doi: 10.1111/j.1558-5646.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- 194. Ekenäs C, Baldwin BG, Andreasen K (2007) A molecular phylogenetic study of Arnica (Asteraceae): Low chloroplast DNA variation and problematic subgeneric classification. Syst Bot 32: 917–928. doi: 10.1043/06-80.1. [DOI] [Google Scholar]
- 195. Clement WL, Donoghue MJ (2012) Barcoding success as a function of phylogenetic relatedness in Viburnum, a clade of woody angiosperms. BMC Evol Biol 12: 73. doi: 10.1186/1471-2148-12-73. PubMed: 22646220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Hoffmann MH, Röser M (2009) Taxon recruitment of the arctic flora: an analysis of phylogenies. New Phytol 182: 774–780. doi: 10.1111/j.1469-8137.2009.02782.x. PubMed: 19309448. [DOI] [PubMed] [Google Scholar]
- 197. Guillon J-M (2004) Phylogeny of horsetails (Equisetum) based on the chloroplast rps4 gene and adjacent noncoding sequences. Syst Bot 29: 251–259. doi: 10.1600/036364404774195467. [DOI] [Google Scholar]
- 198. Guillon JM (2007) Molecular phylogeny of horsetails (Equisetum) including chloroplast atpB sequences. J Plant Res 120: 569–574. doi: 10.1007/s10265-007-0088-x. PubMed: 17476459. [DOI] [PubMed] [Google Scholar]
- 199. Des Marais DL, Smith AR, Britton DM, Pryer KM (2003) Phylogenetic relationships and evolution of extant horsetails, Equisetum, based on chloroplast DNA sequence data (rbcL and trnL‐F). Int J Plant Sci 164: 737–751. doi: 10.1086/37681710.1086 %2F376817 [DOI] [Google Scholar]
- 200. Hauke RL (1962) A resume of the taxonomic reorganization of Equisetum, subgenus Hippochaete, III. Am Fern J 52: 57–63. doi: 10.2307/1546651. [DOI] [Google Scholar]
- 201. Scoggan HJ (1978) The flora of Canada. Part 2. Pteridophyta, Gymospermae, Monocotyledonae. National Museum of Natural Sciences Publications in Botany. p. 545.
- 202. Freeman CC, Levsen N (2007) Chrysosplenium Linnaeus. In: Flora of North America North of Mexico, Volume 8 Magnoliophyta: Paeoniaceae to Ericaceae. Oxford and New York. Oxford University Press; pp. 70–75. [Google Scholar]
- 203. Chinnappa CC, Donald GM, Sasidharan R, Emery RJN (2005) The biology of Stellaria longipes (Caryophyllaceae). Can J Bot 83: 1367–1383. doi: 10.1139/b05-117. [DOI] [Google Scholar]
- 204. Chinnappa CC, Morton JK (1991) Studies on the Stellaria longipes complex (Caryophyllaceae) – Taxonomy. Rhodora 93: 129–135. [Google Scholar]
- 205. Morton JK (2005) Stellaria L. In: Flora of North America North of Mexico, Volume 5 Magnoliophyta: Caryophyllidae, part 2. Oxford and New York: Oxford University Press; pp. 96–114. [Google Scholar]
- 206. Sokoloff PC, Gillespie LJ (2011) Taxonomy of Astragalus robbinsii var. fernaldii (Fabaceae): molecular and morphological analyses support transfer to Astragalus eucosmus . Botany 90: 11–26. doi: 10.1139/b11-077. [DOI] [Google Scholar]
- 207. Drábková L, Kirschner J, Seberg O, Petersen G, Vlcek C (2003) Phylogeny of the Juncaceae based on rbcL sequences, with special emphasis on Luzula DC. and Juncus L. Plant Systematics and Evolution 240: 133–147. .
- 208. Drábková LZ, Vlček Č (2010) Molecular phylogeny of the genus Luzula DC. (Juncaceae, Monocotyledones) based on plastome and nuclear ribosomal regions: A case of incongruence, incomplete lineage sorting and hybridisation. Mol Phylogenet Evol 57: 536–551. doi: 10.1016/j.ympev.2010.07.022. PubMed: 20696260. [DOI] [PubMed] [Google Scholar]
- 209. Drábková L, Kirschner J, Vlček Č (2006) Phylogenetic relationships within Luzula DC. and Juncus L. (Juncaceae): A comparison of phylogenetic signals of trnL-trnF intergenic spacer, trnL intron and rbcL plastome sequence data. Cladistics 22: 132–143. doi: 10.1111/j.1096-0031.2006.00095.x.. [DOI] [PubMed] [Google Scholar]
- 210. Drábková LZ, Vlček Č (2009) DNA variation within Juncaceae: comparison of impact of organelle regions on phylogeny. Plant Syst Evol 278: 169–186. doi: 10.1007/s00606-008-0135-7. [DOI] [Google Scholar]
- 211. Hoot SB, Meyer KM, Manning JC (2012) Phylogeny and reclassification of Anemone (Ranunculaceae), with an emphasis on austral species. Syst Bot 37: 139–152. doi: 10.1600/036364412X616729. [DOI] [Google Scholar]
- 212. Garcia S, McArthur ED, Pellicer J, Sanderson SC, Vallès J et al. (2011) A molecular phylogenetic approach to western North America endemic Artemisia and allies (Asteraceae): Untangling the sagebrushes. Am J Bot 98: 638–653. doi: 10.3732/ajb.1000386. PubMed: 21613164. [DOI] [PubMed] [Google Scholar]
- 213. Wendling BM, Galbreath KE, DeChaine EG (2011) Resolving the evolutionary history of Campanula (Campanulaceae) in western North America. PLOS ONE 6: e23559. doi: 10.1371/journal.pone.0023559. PubMed: 21931605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Carlsen T, Bleeker W, Hurka H, Elven R, Brochmann C (2009) Biogeography and phylogeny of Cardamine (Brassicaceae). Ann Mo Bot Gard 96: 215–236. doi: 10.3417/2007047. [DOI] [Google Scholar]
- 215. Starr JR, Harris SA, Simpson DA (2004) Phylogeny of the unispicate taxa in Cyperaceae tribe Cariceae I: Generic relationships and evolutionary scenarios. Syst Bot 29: 528–544. doi: 10.1600/0363644041744455. [DOI] [Google Scholar]
- 216. Cieslak T, Polepalli JS, White A, Müller K, Borsch T et al. (2005) Phylogenetic analysis of Pinguicula (Lentibulariaceae): chloroplast DNA sequences and morphology support several geographically distinct radiations. Am J Bot 92: 1723–1736. doi: 10.3732/ajb.92.10.1723. PubMed: 21646090. [DOI] [PubMed] [Google Scholar]
- 217. Goetsch L, Eckert AJ, Hall BD, Hoot SB (2005) The molecular systematics of Rhododendron (Ericaceae): A phylogeny based upon RPB2 gene sequences. Syst Bot 30: 616–626. doi: 10.1600/0363644054782170. [DOI] [Google Scholar]
- 218. Greenberg AK, Donoghue MJ (2011) Molecular systematics and character evolution in Caryophyllaceae. Taxon 60: 1637–1652. [Google Scholar]
- 219. Azuma H, Tobe H (2011) Molecular phylogenetic analyses of Tofieldiaceae (Alismatales): family circumscription and intergeneric relationships. J Plant Res 124: 349–357. doi: 10.1007/s10265-010-0387-5. PubMed: 21080207. [DOI] [PubMed] [Google Scholar]
- 220. Eidesen PB, Alsos IG, Popp M, Stensrud Ø, Suda J et al. (2007) Nuclear vs. plastid data: complex Pleistocene history of a circumpolar key species. Mol Ecol 16: 3902–3925. doi: 10.1111/j.1365-294X.2007.03425.x. PubMed: 17850553. [DOI] [PubMed] [Google Scholar]
- 221. Westergaard KB, Alsos IG, Popp M, Engelskjøn T, Flatberg KI et al. (2011) Glacial survival may matter after all: nunatak signatures in the rare European populations of two west-arctic species. Mol Ecol 20: 376–393. doi: 10.1111/j.1365-294X.2010.04928.x. PubMed: 21156004. [DOI] [PubMed] [Google Scholar]
- 222. Alsos IG, Engelskjøn T, Gielly L, Taberlet P, Brochmann C (2005) Impact of ice ages on circumpolar molecular diversity: insights from an ecological key species. Mol Ecol 14: 2739–2753. doi: 10.1111/j.1365-294X.2005.02621.x. PubMed: 16029475. [DOI] [PubMed] [Google Scholar]
- 223. Cherniawsky DM, Bayer RJ (1998) Systematics of North American Petasites (Asteraceae: Senecioneae). II. Isozyme analysis and population genetic structure. Can J Bot 76: 1476–1487. doi: 10.1139/b98-151. [DOI] [Google Scholar]
- 224. Golden JL, Kim YD, Bain JF (2001) A re-evaluation of North American Tephroseris and Sinosenecio (Asteraceae: Senecioneae) based on molecular and micromorphological data. Can J Bot 79: 1195–1201. doi: 10.1139/b01-100. [DOI] [Google Scholar]
- 225. Barkley TM, Murray DM (2006) Tephroseris (Reichenbach) Reichenbach. In: Flora of North America North of Mexico, Volume 20 Magnoliophyta: Asteridae (in part): Asteraceae, part 2. Oxford and New York: Oxford University Press; pp. 541–548. [Google Scholar]
- 226. Bayer RJ, Soltis DE, Soltis PS (1996) Phylogenetic inferences in Antennaria (Asteraceae: Gnaphalieae: Cassiniinae) based on sequences from nuclear ribosomal DNA internal transcribed spacers (ITS). Am J Bot 83: 516–527. doi: 10.2307/244622010.2307 %2F2446220 [DOI] [Google Scholar]
- 227. Bonos SA, Plumley KA, Meyer WA (2002) Ploidy determination in Agrostis using flow cytometry and morphological traits. Crop Sci 42: 192–196. doi: 10.2135/cropsci2002.0192. PubMed: 11756273. [DOI] [PubMed] [Google Scholar]
- 228. Chiapella JO, DeBoer VL, Amico GC, Kuhl JC (2011) A morphological and molecular study in the Deschampsia cespitosa complex (Poaceae; Poeae; Airinae) in northern North America. Am J Bot 98: 1366–1380. doi: 10.3732/ajb.1000495. PubMed: 21821595. [DOI] [PubMed] [Google Scholar]
- 229. Harrison K, Hebda RJ (2011) A morphometric analysis of variation between Elymus alaskanus and Elymus violaceus (Poaceae): implications for recognition of taxa. Madroño 58: 32–49. doi: 10.3120/0024-9637-58.1.32. [DOI] [Google Scholar]
- 230. Sun G, Shee J, Salomon B (2006) Molecular diversity and relationships among Elymus trachycaulus, E. subsecundus, E. virescens, E violaceus, and E. hyperarcticus (Poaceae: Triticeae) as determined by amplified fragment length polymorphism. Genome 49: 1160–1169. 10.1139/g06-062 [DOI] [PubMed] [Google Scholar]
- 231. Ares E, Nurminiemi M, Brochmann C (2000) Incongruent phylogeographies in spite of similar morphology, ecology, and distribution: Phippsia algida and P. concinna (Poaceae) in the North Atlantic region. Plant Syst Evol 220: 241–261. doi: 10.1007/BF00985048. [DOI] [Google Scholar]
- 232. Steen NW, Elven R, Nordal I (2004) Hybrid origin of the arctic x Pucciphippsia vacillans (Poaceae): evidence from Svalbard plants. Plant Syst Evol 245: 215–238. doi: 10.1007/s00606-003-0109-810.1007 %2Fs00606-003-0109-8 [DOI] [Google Scholar]
- 233. Consaul LL, Aiken SG (2007) Phippsia R.Br. In: Barkworth ME, Capels KM, Long S, Anderton LK, Piep MB. Flora of North America, Volume 24 Magnoliophyta: Commelinidae (in part): Poaceae, part 1. Oxford and New York: Oxford University Press; pp. 478–480. [Google Scholar]
- 234. Cräutlein M, Korpelainen H, Pietiläinen M, Rikkinen J (2011) DNA barcoding: a tool for improved taxon identification and detection of species diversity. Biodivers Conserv 20: 373–389. doi: 10.1007/s10531-010-9964-0. [DOI] [Google Scholar]
- 235. Kaplan DR (2008) A taxonomic revision of Stuckenia (Potamogetonaceae) in Asia, with notes on the diversity and variation of the genus on a worldwide scale. Folia Geobotanica 43: 159–234. doi: 10.1007/s12224-008-9010-0. [DOI] [Google Scholar]
- 236. Brysting AK, Elven R (2000) The Cerastium alpinum-C. arcticum complex (Caryophyllaceae): numerical analyses of morphological variation and a taxonomic revision of C. arcticum Lange s.l. Taxon 49: 189–216. doi: 10.2307/1223835. [DOI] [Google Scholar]
- 237. Scheen AC, Brochmann C, Brysting AK, Elven R, Morris A et al. (2004) Northern hemisphere biogeography of Cerastium (Caryophyllaceae): insights from phylogenetic analysis of noncoding plastidnucleotide sequences. Am J Bot 91: 943–952. doi: 10.3732/ajb.91.6.943. PubMed: 21653450. [DOI] [PubMed] [Google Scholar]
- 238. Ferren WR Jr., Schenk HJ (2004) Suaeda Forsskål ex J. F. Gmelin. In: Flora of North America Editorial Committee, editor. Flora of North America North of Mexico Volume 4 Magnoliophyta: Caryophyllidae, Part 1. Oxford and New York: Oxford University Press. [Google Scholar]
- 239. Kapralov MV, Akhani H, Voznesenskaya EV, Edwards G, Franceschi V et al. (2006) Phylogenetic relationships in the Salicornioideae / Suaedoideae / Salsoloideae s.l. (Chenopodiaceae) clade and a clarification of the phylogenetic position of Bienertia and Alexandra using multiple DNA sequence datasets. Syst Bot 31: 571–585. doi: 10.1043/06-01.1. [DOI] [Google Scholar]
- 240. Guggisberg A, Mansion G, Kelso S, Conti E (2006) Evolution of biogeographic patterns, ploidy levels, and breeding systems in a diploid–polyploid species complex of Primula . New Phytol 171: 617–632. doi: 10.1111/j.1469-8137.2006.01722.x. PubMed: 16866963. [DOI] [PubMed] [Google Scholar]
- 241. Davis JI (1983) Phenotypic plasticity and the selection of taxonomic characters in Puccinellia (Poaceae). Syst Bot 8: 341–353. doi: 10.2307/241835410.2307 %2F2418354 [DOI] [Google Scholar]
- 242. Aiken SG, Consaul LL, Davis JI, Manos PS (1993) Systematic inferences from variation in isozyme profiles of arctic and alpine cespitose Festuca (Poaceae). Am J Bot 80: 76–82. doi: 10.2307/244512210.2307 %2F2445122 [DOI] [Google Scholar]
- 243. Darbyshire SJ, Pavlick LE (2007) Festuca L. In: Barkworth ME, Capels KM, Long S, Anderton LK, Piep MB. Flora of North America, Volume 24 Magnoliophyta: Commelinidae (in part): Poaceae, part 1. Oxford and New York: Oxford University Press; pp. 389–443. [Google Scholar]
- 244. Catalán P, Torrecilla P, Rodrı ´guez JÁL, Olmstead RG (2004) Phylogeny of the festucoid grasses of subtribe Loliinae and allies (Poeae, Pooideae) inferred from ITS and trnL–F sequences. Mol Phylogenet Evol 31: 517–541. doi: 10.1016/j.ympev.2003.08.025. PubMed: 15062792. [DOI] [PubMed] [Google Scholar]
- 245. Aiken SG, Darbyshire SJ (1990) Fescue Grasses of Canada. Ottawa: Agriculture Canada. 113 p. doi: 10.5962/bhl.title.59072. [DOI]
- 246. Fjellheim S, Elven R, Brochmann C (2001) Molecules and morphology in concert. II. The Festuca brachyphylla complex (Poaceae) in Svalbard. Am J Bot 88: 869–882. doi: 10.2307/2657039. PubMed: 1135371210.2307%2F2657039 [DOI] [PubMed] [Google Scholar]
- 247. Guldahl AS, Borgen L, Nordal I (2008) Variation in the Festuca brachyphylla (Poaceae) complex in Svalbard, elucidated by chromosome numbers and isozymes. Bot J Linn Soc 137: 107–126. doi: 10.1111/j.1095-8339.2001.tb01112.x. [DOI] [Google Scholar]
- 248. Pavlick LE (1984) Studies on the Festuca ovina complex in the Canadian Cordillera. Can J Bot 62: 2448–2462. doi: 10.1139/b84-334. [DOI] [Google Scholar]
- 249. Aiken SG, Lefkovitch LP, Darbyshire SJ, Armstrong KC (1988) Vegetative proliferation in inflorescences of red fescue (Festuca rubra s.l., Poaceae). Can J Bot 66: 1–10. doi: 10.1139/b88-001. [DOI] [Google Scholar]
- 250. Chiurugwi T, Beaumont MA, Wilkinson MJ, Battey NH (2011) Adaptive divergence and speciation among sexual and pseudoviviparous populations of Festuca . Heredity 106: 854–861. doi: 10.1038/hdy.2010.128. PubMed: 20959864. Retrieved onpublished at whilst December year 1111 from http://www.nature.com/hdy/journal/v106/n5/suppinfo/hdy2010128s1.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Soreng RJ (2007) Poa L. In: Barkworth ME, Capels KM, Long S, Anderton LK, Piep MB. Flora of North America, Volume 24 Magnoliophyta: Commelinidae (in part): Poaceae, part 1. Oxford and New York: Oxford University Press; pp. 486–601. [Google Scholar]
- 252. Gillespie LJ, Soreng RJ (2005) A phylogenetic analysis of the bluegrass genus Poa based on cpDNA restriction site data. Syst Bot 30: 84–105. doi: 10.1600/0363644053661940. [DOI] [Google Scholar]
- 253. Cowan RS, Chase MW, Kress WJ, Savolainen V (2006) 300,000 species to identify: problems, progress, and prospects in DNA barcoding of land plants. Taxon 55: 611–616. doi: 10.2307/25065638. [DOI] [Google Scholar]
- 254. Ree RH (2005) Phylogeny and the evolution of floral diversity in Pedicularis (Orobanchaceae). Int J Plant Sci 166: 595–613. doi: 10.1086/430191. [DOI] [Google Scholar]
- 255. Argus GW (2007) Salix (Salicaceae) distribution maps and a synopsis of their classification in North America, north of Mexico. Harv Pap Bot 12: 335–368. doi:10.3100/1043-4534(2007)12[335:SSDMAA]2.0.CO;2. [Google Scholar]
- 256. Chen J-H, Sun H, Wen J, Yang Y-P (2010) Molecular phylogeny of Salix L. (Salicaceae) inferred from three chloroplast datasets and its systematic implications. Taxon 59: 29–37. [Google Scholar]
- 257. Azuma T, Kajita T, Yokoyama J, Ohashi H (2000) Phylogenetic relationships of Salix (Salicaceae) based on rbcL sequence data. Am J Bot 87: 67–75. doi: 10.2307/2656686. PubMed: 1063683110.2307%2F2656686 [DOI] [PubMed] [Google Scholar]
- 258. Hardig TM, Antilla CK, Brunsfeld SJ (2010) A phylogenetic analysis of Salix (Salicaceae) based on matK and ribosomal DNA sequence data. J of Bot: 2010: Article ID 197696 doi: 10.1155/2010/197696. [DOI] [Google Scholar]
- 259. Jordon-Thaden I, Hase I, Al-Shehbaz I, Koch MA (2010) Molecular phylogeny and systematics of the genus Draba (Brassicaceae) and identification of its most closely related genera. Mol Phylogenet Evol 55: 524–540. doi: 10.1016/j.ympev.2010.02.012. PubMed: 20170737. [DOI] [PubMed] [Google Scholar]
- 260. Conti E, Soltis DE, Hardig TM, Schneider J (1999) Phylogenetic relationships of the silver saxifrages (Saxifraga, Sect. Ligulatae Haworth): Implications for the evolution of substrate specificity, life histories, and biogeography. Mol Phylogenet Evol 13: 536–555. doi: 10.1006/mpev.1999.0673. PubMed: 1062041210.1006%2Fmpev.1999.0673 [DOI] [PubMed] [Google Scholar]
- 261. Brouillet L, Elvander PE (2009) Saxifraga Linnaeus. In: Flora of North America North of Mexico, Volume 8 Magnoliophyta: Paeoniaceae to Ericaceae. Oxford and New York. Oxford University Press; pp. 132–146. [Google Scholar]
- 262. Jørgensen MH, Elven R, Tribsch A, Gabrielsen TM, Stedje B et al. (2006) Taxonomy and evolutionary relationships in the Saxifraga rivularis complex. Syst Bot 31: 702–729. doi: 10.1600/03636440677969598810.1600 %2F036364406779695988 [DOI] [Google Scholar]
- 263. Brochmann C, Xiang Q, Brunsfeld S, Soltis D, Soltis P (1998) Molecular evidence for polyploid origins in Saxifraga (Saxifragaceae): the narrow arctic endemic S. svalbardensis and its widespread allies. Am J Bot 85: 135. doi: 10.2307/2446562. PubMed: 2168488710.2307%2F2446562 [DOI] [PubMed] [Google Scholar]
- 264. Whittemore AT (1997) Ranunculus L. In: Flora of North America North of Mexico, Volume 3 Magnoliophyta: Magnoliidae – Hamamelidae. Oxford and New York. Oxford University Press; pp. 88–135. [Google Scholar]
- 265. Paun O, Lehnebach C, Johansson JT, Lockhart P, Hörandl E (2005) Phylogenetic relationships and biogeography of Ranunculus and allied genera (Ranunculaceae) in the Mediterranean region and in the European Alpine System. Taxon 54: 911–930. doi: 10.2307/2506547810.2307 %2F25065478 [DOI] [Google Scholar]
- 266. Hoffmann MH, von Hagen KB, Hörandl E, Röser M, Tkach NV (2010) Sources of the arctic flora: origins of arctic species in Ranunculus and related genera. Int J Plant Sci 171: 90–106. doi: 10.1086/647918. PubMed: 20582248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Emadzade K, Gehrke B, Peter Linder H, Hörandl E (2011) The biogeographical history of the cosmopolitan genus Ranunculus L. (Ranunculaceae) in the temperate to meridional zones. Molecular Phylogenetics and Evolution 58: 4–21. 10.1016/j.ympev.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 268. Hörandl E, Emadzade K (2011) The evolution and biogeography of alpine species in Ranunculus (Ranunculaceae): A global comparison. Taxon 60: 415–426. [Google Scholar]
- 269. Hörandl E, Emadzade K; (2012) Evolutionary classification: A case study on the diverse plant genus Ranunculus L (Ranunculaceae: ). Perspectives in Plant Ecology, Evolution and Systematics 14: 310–324 doi: 10.1016/j.ppees.2012.04.001. [DOI] [Google Scholar]
- 270. Scott PJ (1974) The systematics of Ranunculus gmelinii and R. hyperboreus in North America. Can J Bot 52: 1713–1722. doi: 10.1139/b74-223. [DOI] [Google Scholar]
- 271. Dobes C, Paule J (2010) A comprehensive chloroplast DNA-based phylogeny of the genus Potentilla (Rosaceae): Implications for its geographic origin, phylogeography and generic circumscription. Mol Phylogenet Evol 56: 156–175. doi: 10.1016/j.ympev.2010.03.005. PubMed: 20214995. [DOI] [PubMed] [Google Scholar]
- 272. Töpel M, Lundberg M, Eriksson T, Eriksen B (2011) Molecular data and ploidal levels indicate several putative allopolyploidization events in the genus Potentilla (Rosaceae). PLOS Currents Tree of Life 2011 May 18 [last modified: 2012 Apr 4] Edition 1: doi: 10.1371/currents.RRN1237. [DOI] [PMC free article] [PubMed]
- 273. Soják J (2010) Argentina Hill, a genus distinct from Potentilla (Rosaceae). Thaiszia J Bot 20: 91–87. [Google Scholar]
- 274. Nyléhn J, Hamre E, Nordal I (2003) Facultative apomixis and hybridization in arctic Potentilla section Niveae (Rosaceae) from Svalbard. Bot J Linn Soc 142: 373–381. doi: 10.1046/j.1095-8339.2003.00190.x. [DOI] [Google Scholar]
- 275. Hansen KT, Elven R, Brochmann C (2000) Molecules and morphology in concert: tests of some hypotheses in arctic Potentilla (Rosaceae). Am J Bot 87: 1466–1479. doi: 10.2307/2656873. PubMed: 1103492210.2307%2F2656873 [DOI] [PubMed] [Google Scholar]
- 276. Eriksen B, Töpel MH (2006) Molecular phylogeography and hybridization in members of the circumpolar Potentilla sect. Niveae (Rosaceae). Am J Bot 93: 460–469. doi: 10.3732/ajb.93.3.460. PubMed: 21646205. [DOI] [PubMed] [Google Scholar]
- 277. Dobe Š, Scheffknecht S (2012) Isolation and characterization of microsatellite loci for the Potentilla core group (Rosaceae) using 454 sequencing. Mol Ecol Resour 12: 726–739. doi: 10.1111/j.1755-0998.2012.03134.x. PubMed: 22463760. [DOI] [PubMed] [Google Scholar]
- 278. Starr JR, Ford BA (2009) Phylogeny and evolution in Cariceae (Cyperaceae): Current knowledge and future directions. Bot Rev 75: 110–137. doi: 10.1007/s12229-008-9020-x. [DOI] [Google Scholar]
- 279. Waterway MJ, Starr JR (2007) Phylogenetic relationships in tribe Cariceae (Cyperaceae) based on nested analyses of four molecular datasets. Aliso 23: 103–130. [Google Scholar]
- 280. Cochrane TS (2002) Carex Linnaeus sect. Physoglochin Dumortier. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 299–301. [Google Scholar]
- 281. Reznicek AA, Catling PM (2002) Carex Linnaeus sect. Chordorrhizae (Heuffel) Meinshausen. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 298–299. [Google Scholar]
- 282. Reznicek AA (2002) Carex Linnaeus sect. Foetidae (Tuckerman ex LH Bailey) Kükenthal. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 309–311. [Google Scholar]
- 283. Cochrane TS (2002) Carex Linnaeus sect. Heleoglochin Dumortier. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 278–281. [Google Scholar]
- 284. Toivonen H (2002) Carex Linnaeus sect. Glareosae G Don. In: Commitee, editor, Flora of North America Editorial. Flora of North America North of Mexico, Vol 23: Magnoliophyta; Commelinidae (in part): Cyperaceae. Oxford: and New York: Oxford University Press; . pp. 311–321 [Google Scholar]
- 285. King MG, Roalson EH (2008) Exploring evolutionary dynamics of nrDNA in Carex subgenus Vignea (Cyperaceae). Syst Bot 33: 514–524. doi: 10.1600/036364408785679860. [DOI] [Google Scholar]
- 286. Ford BA, Iranpour M, Naczi RFC, Starr JR, Jerome CA (2006) Phylogeny of Carex subg. Vignea (Cyperaceae) based on non-coding nrDNA sequence data. Syst Bot 31: 70–82. doi: 10.1600/036364406775971813. [DOI] [Google Scholar]
- 287. Ford BA, Ghazvini H, Naczi RFC, Starr JR (2012) Phylogeny of Carex subg. Vignea (Cyperaceae) based on amplified fragment length polymorphism and nrDNA data. Syst Bot 37: 913–925. doi: 10.1600/036364412X656464. [DOI] [Google Scholar]
- 288. Escudero M, Valcárcel V, Vargas P, Luceño M (2010) Bipolar disjunctions in Carex: Long-distance dispersal, vicariance, or parallel evolution? Flora 205: 118–127. doi: 10.1016/j.flora.2009.01.00510.1016 %2Fj.flora.2009.01.005 [DOI] [Google Scholar]
- 289. Starr JR, Harris SA, Simpson DA (2008) Phylogeny of the unispicate taxa in Cyperaceae tribe Cariceae II: the limits of Uncinia . In: Naczi RFC, Ford BA. Sedges: uses, diversity, and systematics of the Cyperaceae. pp. 243–267. [Google Scholar]
- 290. Reznicek AA, Ford BA (2002) Carex Linnaeus sect. Vesicariae (Heuffel) J. Carey. In: Flora of North America Editorial Committee, editor. Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York: Oxford University Press; . pp. 501–511 [Google Scholar]
- 291. Shekhovtsov SV, Shekhovtsova IN, Peltek SE (2012) Phylogeny of Siberian species of Carex sect. Vesicariae based on nuclear and plastid markers. Nord J Bot 30: 343–351. doi: 10.1111/j.1756-1051.2011.01405.x. [DOI] [Google Scholar]
- 292. Standley LA, Cayouette J, Bruederle LP (2002) Carex Linnaeus sect. Phacocystis Dumortier. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. New York: Oxford University Press; pp. 379–401. [Google Scholar]
- 293. Schönswetter P, Elven R, Brochmann C (2008) Trans-Atlantic dispersal and large-scale lack of genetic structure in the circumpolar, arctic-alpine sedge Carex bigelowii s. l. (Cyperaceae). Am J Bot 95: 1006–1014. doi: 10.3732/ajb.2007196. PubMed: 21632421. [DOI] [PubMed] [Google Scholar]
- 294. Dragon JA, Barrington DS (2008) East vs. West: Monophyletic clades within the paraphyletic Carex acuta complex, section Phacocystis (Cyperaceae). In: Naczi RFC, Ford BA. Sedges: uses, diversity, and systematics of the Cyperaceae. pp. 215–226. [Google Scholar]
- 295. Dragon JA, Barrington DS (2009) Systematics of the C. aquatilis and C. Lenticularis lineages: Geographically and ecologically divergent sister clades of Carex section Phacocystis (Cyperaceae). Am J Bot 96: 1896–1906. doi: 10.3732/ajb.0800404. PubMed: 21622311. [DOI] [PubMed] [Google Scholar]
- 296. Murray DM (2002) Carex Linnaeus sect. Scitae Kukenthal. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 414–416. [Google Scholar]
- 297. Murray DF (2002) Carex Linnaeus sect. Racemosae G Don. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 401–414. [Google Scholar]
- 298. Ball PW (2002) Carex Linnaeus sect. Bicolores (Tuckerman ex LH Bailey) Rouy. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 424–426. [Google Scholar]
- 299. Rothrock PE, Reznicek AA (2002) Carex Linnaeus sect. Paniceae G Don. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 426–431. [Google Scholar]
- 300. Ball PW (2002) Carex Linnaeus sect. Limosa (Heuffel) Meinshausen. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 416–419. [Google Scholar]
- 301. Dunlop DA (2002) Carex Linnaeus sect. Scirpinae (Tuckerman) Kükenthal. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 549–553. [Google Scholar]
- 302. Crins WJ (2002) Carex Linnaeus sect. Clandestinae GDon. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 546–548. [Google Scholar]
- 303. Ball PW, Murray DM (2002) Carex Linnaeus sect. Lamprochlaenae (Drejer) L.H Bailey. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 556–557. [Google Scholar]
- 304. Ball PW (2002) Carex Linnaeus sect. Chlorostachyae Tuckerman ex Meinshausen. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 475–477. [Google Scholar]
- 305. Ball PW, Mastrogiuseppe J (2002) Carex Linnaeus sect. Aulocystis Dumortier. In: Flora of North America North of Mexico, Vol 23: Magnoliophyta: Commelinidae (in part): Cyperaceae. Oxford and New York. Oxford University Press; pp. 477–482. [Google Scholar]
- 306. Hendrichs M, Oberwinkler F, Begerow D, Bauer R (2004) Carex, subgenus Carex (Cyperaceae)-A phylogenetic approach using ITS sequences. Plant Syst Evol 246: 89–107. doi: 10.1007/s00606-004-0128-0. [DOI] [Google Scholar]
- 307. Richards AJ (1973) The origin of Taraxacum agamospecies. Bot J Linn Soc 66: 189–211. doi: 10.1111/j.1095-8339.1973.tb02169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Kirschner J, Štěpánek J (1997) A nomenclatural checklist of supraspecific names in Taraxacum . Taxon 46: 87–98. doi: 10.2307/1224294. [DOI] [Google Scholar]
- 309. Wittzell H (1999) Chloroplast DNA variation and reticulate evolution in sexual and apomictic sections of dandelions. Mol Ecol 8: 2023–2035. doi: 10.1046/j.1365-294x.1999.00807.x. PubMed: 10632854. [DOI] [PubMed] [Google Scholar]
- 310. Kirschner J, Štěpánek J (1996) Modes of speciation and evolution of the sections in Taraxacum . Folia Geobotanica Phytotaxonomica 31: 415–426. doi: 10.2307/4181475. [DOI] [Google Scholar]
- 311. Kirschner J, Štěpánek J, Mes THM, Nijs; JCMd, Oosterveld P et al. (2003) Principal features of the cpDNA evolution in Taraxacum (Asteraceae, Lactuceae): a conflict with taxonomy. Plant Syst Evol 239: 231–255. doi: 10.1007/s00606-003-0002-5. 10.1007/s00606-003-0002-5 [DOI] [Google Scholar]
- 312. King LM (1993) Origins of genotypic variation in North American dandelions inferred from ribosomal DNA and chloroplast DNA restriction enzyme analysis. Evolution 47: 136–151. doi: 10.2307/2410124. [DOI] [PubMed] [Google Scholar]
- 313. Majeský Ľ, Vašut RJ, Kitner M, Trávníček B (2012) The pattern of genetic variability in apomictic clones of Taraxacum officinale indicates the alternation of asexual and sexual histories of apomicts. PLOS ONE 7: e41868. doi: 10.1371/journal.pone.0041868. PubMed: 22870257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Brouillet L (2006) Taraxacum FH Wiggers. In: Flora of North America North of Mexico, Volume 19 Magnoliophyta: Asteridae (in part): Asteraceae, part 1. Oxford and New York: Oxford University Press; pp. 239–252. [Google Scholar]
- 315. Kirschner J, Štěpánek J (1994) Clonality as a part of the evolution process in Taraxacum . Folia Geobotanica Phytotaxonomica 29: 265–275. doi: 10.2307/4181272. [DOI] [Google Scholar]
- 316. Brock MT (2004) The potential for genetic assimilation of a native dandelion species, Taraxacum ceratophorum (Asteraceae), by the exotic congener T. officinale . Am J Bot 91: 656–663. doi: 10.3732/ajb.91.5.656. PubMed: 21653420. [DOI] [PubMed] [Google Scholar]
- 317. Harbaugh DT, Nepokroeff M, Rabeler RK, McNeill J, Zimmer EA et al. (2010) A new lineage-based tribal classification of the family Caryophyllaceae. Int J Plant Sci 171: 185–198. doi: 10.1086/648993. [DOI] [Google Scholar]
- 318. Wolf SJ, Packer J, Denford K (1979) The taxonomy of Minuartia rossii (Caryophyllaceae). Can J Bot 57: 1673–1686. doi: 10.1139/b79-205. [DOI] [Google Scholar]
- 319. Abbott RJ, Brochmann C (2003) History and evolution of the arctic flora: in the footsteps of Eric Hultén. Mol Ecol 12: 299–313. doi: 10.1046/j.1365-294X.2003.01731.x. PubMed: 12535083. [DOI] [PubMed] [Google Scholar]
- 320. Marr KL, Allen GA, Hebda RJ (2008) Refugia in the Cordilleran ice sheet of western North America: chloroplast DNA diversity in the Arctic–alpine plant Oxyria digyna . J Biogeogr 35: 1323–1334. doi: 10.1111/j.1365-2699.2007.01879.x. [DOI] [Google Scholar]
- 321. Allen GA, Marr KL, McCormick LJ, Hebda RJ (2012) The impact of Pleistocene climate change on an ancient arctic–alpine plant: multiple lineages of disparate history in Oxyria digyna . Ecol Evolution 2: 649–665. doi: 10.1002/ece3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Powell EA, Kron KA (2002) Hawaiian blueberries and their relatives—a phylogenetic analysis of Vaccinium sections Macropelma, Myrtillus, and Hemimyrtillus (Ericaceae). Syst Bot 27: 768–779. doi: 10.1043/0363-6445-27.4.768. [DOI] [Google Scholar]
- 323. Anderberg AA, Rydin C, Källersjö M (2002) Phylogenetic relationships in the order Ericales s.l.: analyses of molecular data from five genes from the plastid and mitochondrial genomes. Am J Bot 89: 677–687. doi: 10.3732/ajb.89.4.677. PubMed: 21665668. [DOI] [PubMed] [Google Scholar]
- 324. Abbott RJ, Comes HP (2004) Evolution in the Arctic: a phylogeographic analysis of the circumarctic plant, Saxifraga oppositifolia (Purple saxifrage). New Phytol 161: 211–224. doi: 10.1046/j.1469-8137.2003.00953.x. [DOI] [Google Scholar]
- 325. Abbott RJ, Smith LC, Milne RI, Crawford RMM, Wolff K et al. (2000) Molecular analysis of plant migration and refugia in the arctic. Science 289: 1343–1346. doi: 10.1126/science.289.5483.1343. PubMed: 10958779. [DOI] [PubMed] [Google Scholar]
- 326. Holderegger R, Abbott RJ (2003) Phylogeography of the Arctic-Alpine Saxifraga oppositifolia (Saxifragaceae) and some related taxa based on cpDNA and ITS sequence variation. Am J Bot 90: 931–936. doi: 10.3732/ajb.90.6.931. PubMed: 21659189. [DOI] [PubMed] [Google Scholar]
- 327. Winkler M, Tribsch A, Schneeweiss GM, Brodbeck S, Gugerli F et al. (2012) Tales of the unexpected: Phylogeography of the arctic-alpine model plant Saxifraga oppositifolia (Saxifragaceae) revisited. Mol Ecol 21: 4618–4630. doi: 10.1111/j.1365-294X.2012.05705.x. PubMed: 22809067. [DOI] [PubMed] [Google Scholar]
- 328. Jørgensen T, Kjær KH, Haile J, Rasmussen M, Boessenkool S et al. (2012) Islands in the ice: detecting past vegetation on Greenlandic nunataks using historical records and sedimentary ancient DNA Meta-barcoding. Mol Ecol 21: 1980–1988. doi: 10.1111/j.1365-294X.2011.05278.x. PubMed: 21951625. [DOI] [PubMed] [Google Scholar]
- 329. CBOL Plant Working Group CBOL Plant Working Group, Hollingsworth PM, Forrest LL, Spouge JL, Hajibabaei M, et al. (2009) A DNA barcode for land plants. Proc Natl Acad Sci USA 106: 12794-12797. doi: 10.1073/pnas.0905845106. PubMed: 19666622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Voucher specimen information, including the scientific names of taxa sampled, locality information, collection dates, collectors and collection numbers, herbarium accession numbers, and GenBank accession numbers.
(XLSX)
Species with two or more infraspecific taxa sampled, and the ability of rbcL, matK, and rbcL + matK to resolve the conspecific infraspecific taxa.
(PDF)
Statistics on the recovery of matK from samples obtained from silica-gel dried leaf material.
(PDF)
Ability of the supplementary plastid DNA barcode loci psbA–trnH, psbK–I and atpF–atpH to discriminate species and infraspecific taxa of Poa and Puccinellia.
(PDF)
Species from the Canadian Arctic Archipelago for which barcode data were. not obtained in the current study.
(PDF)
Frequency histogram showing the distribution of the number of individuals sampled per species. Putative hybrids are not included.
(PDF)
Numbers of rbcL and matK sequences recovered from each plant family. (A) Families with less than 50 samples recovered per family. (B) Families with more than 50 samples recovered per family.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Amaranthaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Apiaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Asteraceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Betulaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analysis of uncorrected p-distances of matK sequence data for Boraginaceae.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Brassicaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Campanulaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Caprifoliaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Caryophyllaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Celastraceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Cyperaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Diapensiaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analysis of uncorrected p-distances of rbcL sequence data for Dryopteridaceae.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Elaeagnaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analysis of uncorrected p-distances of rbcL sequence data for Equisetaceae.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Ericaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Fabaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Gentianaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analysis of uncorrected p-distances of rbcL sequence data for Haloragaceae.
(PDF)
Neighbour joining analysis of uncorrected p-distances of rbcL sequence data for Juncaceae.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Juncaginaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Lentibulariaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL sequence data for Linaceae.
(PDF)
Neighbour joining analysis of uncorrected p-distances of rbcL sequence data for Lycopodiaceae.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Melanthiaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Menyanthaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Montiaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Onagraceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Orchidaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Orobanchaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Papaveraceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analysis of uncorrected p-distances of rbcL sequence data for Pinales (Pinaceae, Cupressaceae).
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Plantaginaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Plumbaginaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Poaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Polemoniaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Polygonaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Potamogetonaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Primulaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Ranunculaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Rosaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Salicaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Saxifragaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Tofieldiaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analyses of uncorrected p-distances of rbcL and matK sequence data for Typhaceae. A. rbcL. B. matK. C. rbcL + matK.
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Puccinellia (Poaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of psbA–trnH sequence data for Puccinellia and Poa (Poaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of atpF–atpH sequence data for Puccinellia and Poa (Poaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of psbK–psbI sequence data for Puccinellia and Poa (Poaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Festuca (Poaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Poa (Poaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Pedicularis (Orobanchaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Salix (Salicaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Draba (Brassicaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Saxifraga (Saxifragaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Ranunculus (Ranunculaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Potentilla (Rosaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of combined rbcL + matK sequence data for Carex (Cyperaceae).
(PDF)
Neighbour joining analysis of uncorrected p-distances of matK sequence data for Carex and Kobresia (Cyperaceae). The tree combines data from the current study and previously published data from Le Clerc-Blain et al. [108], the latter prefaced with the project code "CAREX". .
(PDF)