Abstract
Background
Tools for quantification of asthma severity are limited.
Objective
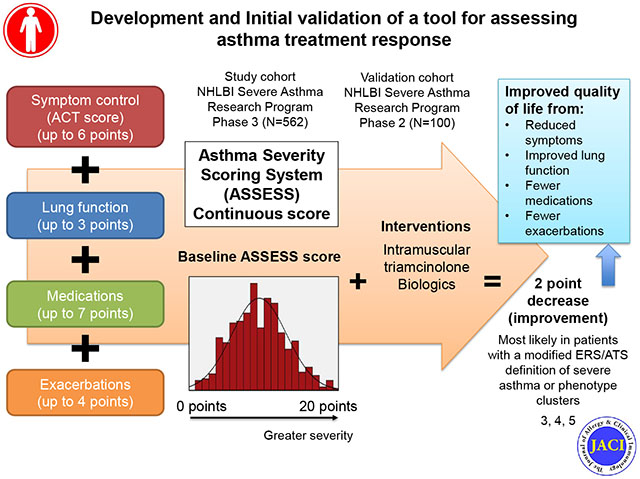
To develop a continuous measure of asthma severity, the Asthma Severity Scoring System (ASSESS), for adolescents and adults incorporating domains of asthma control, lung function, medications, and exacerbations.
Methods
Baseline and 36-month longitudinal data from participants in Phase 3 of the Severe Asthma Research Program (SARP, ) were utilized. Scale properties, responsiveness, and a minimal important difference (MID) were determined. External replication was performed in participants enrolled in SARP Phase 1/2. Utility of ASSESS for detecting treatment response was explored in participants undergoing corticosteroid responsiveness testing with intramuscular triamcinolone and participants receiving biologics.
Results
ASSESS scores ranged from 0 to 20 (8.78 ± 3.9; higher scores reflect worse severity) and differed between 5 phenotypic groups. Measurement properties were acceptable. ASSESS was responsive to changes in quality of life with a MID of 2, with good specificity for outcomes of asthma improvement and worsening, but poor sensitivity. Replication analyses yielded similar results, with a 2-point decrease (improvement) associated with improvements in quality of life. Participants with ≥2 point decrease (improvement) in ASSESS scores also had greater improvement in lung function and asthma control after triamcinolone, but these differences were limited to phenotypic clusters 3, 4 and 5. Participants treated with biologics also had ≥2 point decrease (improvement) in ASSESS scores overall.
Conclusions
The ASSESS tool is an objective measure that may be useful in epidemiologic and clinical research studies for quantification of treatment response in individual patients and in phenotypic groups. However, validation studies are warranted.
Keywords: Asthma control, Asthma severity classification, Severe asthma, Psychometric testing, Tool development
Graphical Abstract
Capsule summary
Objective tools for quantifying asthma severity are limited. The Asthma Severity Scoring System (ASSESS) assesses 4 domains of asthma and may be useful for quantifying treatment responses in research settings.
Introduction
Asthma severity is a unique construct, independent of asthma control, that encompasses a range of asthma patients from mild to severe.1,2 Whereas asthma “control” refers to the extent to which symptoms and other features of asthma are present in patients, asthma “severity” reflects the level of treatment required to control symptoms and exacerbations.2 Like asthma control, asthma severity is not a static feature and can change over the course of months or years in response to seasonal variations, infections and other exposures.3–5 However, while there are many standardized tools available for the assessment of asthma control in both clinical and research settings, tools for quantification of asthma severity are not widely available.
The National Institute of Allergy and Infectious Disease-sponsored Inner-City Asthma Consortium recently developed a Composite Asthma Severity Index (CASI) which quantifies asthma severity through domains of impairment, risk, and medication requirements, but that tool was developed in a population of children (and adolescents) and lacks details on systemic corticosteroids, emerging controller medications such as tiotropium, and biologic therapies that are currently utilized for the treatment of severe asthma.2 Recognizing that severe asthma is a multidimensional construct, we aimed to develop a continuous measure of asthma severity for adolescents and adults that: 1) incorporates fundamental aspects of asthma control, lung function, current controller medications, and recent exacerbations in the assessment of the construct without extensive questioning, 2) is sensitive to short-term temporal changes in asthma and its management, 3) does not require prior knowledge of the patient, and 4) could be utilized by future investigators as well as clinicians in real-world practice settings. The development, measurement properties, and validation of this tool, named the “ASthma Severity Scoring System” (ASSESS), are reviewed below.
Methods
Participants age 12 and older enrolled in Phase 3 of the National Heart, Lung and Blood Institute’s Severe Asthma Research Program (SARP) between November 2012 and February 2015 with at least one year of follow-up data were included in the analysis (Figure E1). Eligibility criteria for SARP3 included a physician diagnosis of asthma and either ≥ 12% reversibility in the forced expiratory volume in one second (FEV1) after bronchodilator administration or airway hyperresponsiveness to methacholine, evidenced by a provocative concentration of methacholine ≤ 16 mg/mL. Thirteen participants with FEV1 values below 50% of predicted who could not undergo methacholine challenge due to concerns for safety were also enrolled at the discretion of the principal investigator. Current smoking, smoking history >10 pack years if ≥30 years of age or >5 pack years if <30 years of age, premature birth before 35 weeks gestation, and other chronic airway disorders such as aspiration or vocal cord dysfunction were criteria for exclusion. Permission to proceed with SARP studies was granted by the Institutional Review Board of each institution and an independent Data Safety and Monitoring Board. The study was also registered at ClinicalTrials.gov (). Informed written consent and assent (if less than 18 years) were obtained from all participants prior to enrollment in these studies.
SARP design and procedures.
Details of the study design have been reported previously.6, 7 Participants completed a baseline characterization visit during which systemic triamcinolone acetonide was administered, a follow-up visit at 18 ± 3 days to assess triamcinolone response, and follow-up characterization visits at 12, 24 and 36 months (±90 days). Visits were postponed if an asthma exacerbation treated with systemic corticosteroids or a respiratory infection treated with antibiotics was reported within the preceding four or two weeks, respectively. Telephone calls were completed every 6 months (±60 days) to assess for asthma control and healthcare utilization. Exacerbations were defined according to a consensus report8 as a worsening of asthma necessitating treatment with systemic corticosteroids. Severe asthma was defined using a modification of the European Respiratory Society (ERS)/American Thoracic Society (ATS) definition9 that required the presence of: 1) asthma requiring Global Initiative for Asthma (GINA) step 4-5 treatment with high doses of inhaled corticosteroids (ICS) and other controller medications, or 2) systemic corticosteroid treatment for ≥50% of the previous year to achieve or maintain asthma control. Management by an asthma specialist was not a criterion for severe asthma in SARP3. Other SARP3 characterization procedures including assignment of participants to 5 phenotypic asthma clusters previously identified in Phase 1 and Phase 2 of SARP10 are detailed in the online repository.
Tool development.
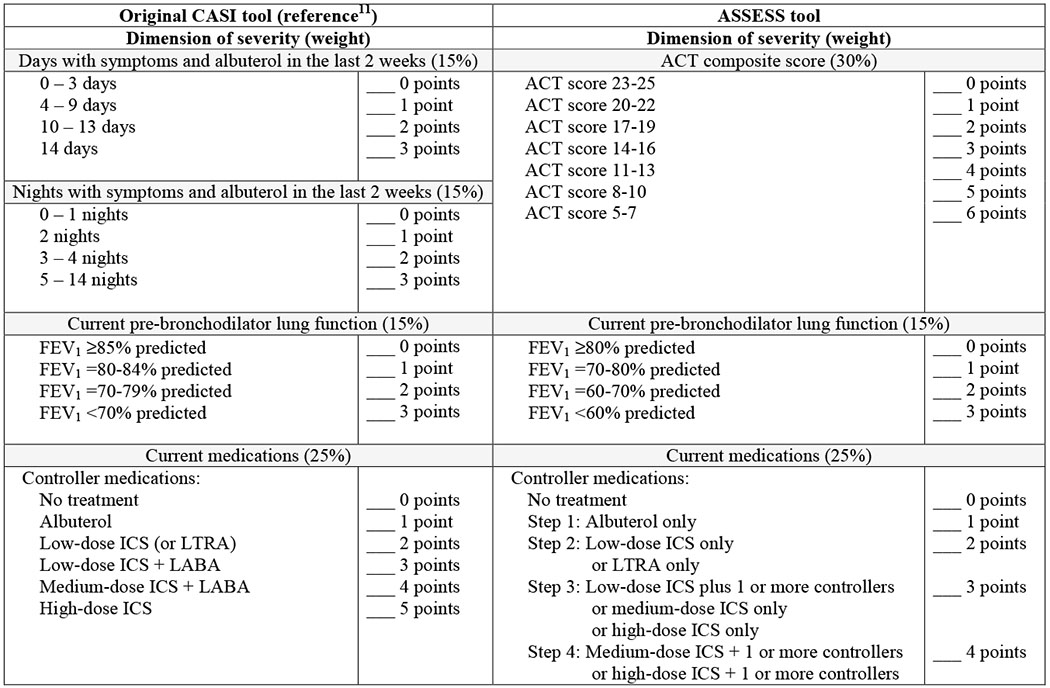
ASSESS is an adaptation of the CASI,11 which was developed using the Delphi method and tested with data from children enrolled in a clinical trials network (Table 1). Details of the ASSESS tool development are provided in the online repository and in Tables E1–E3 and Figure E2.
Table 1.
Asthma Severity Scoring System (ASSESS) for age 12 and older.
 |
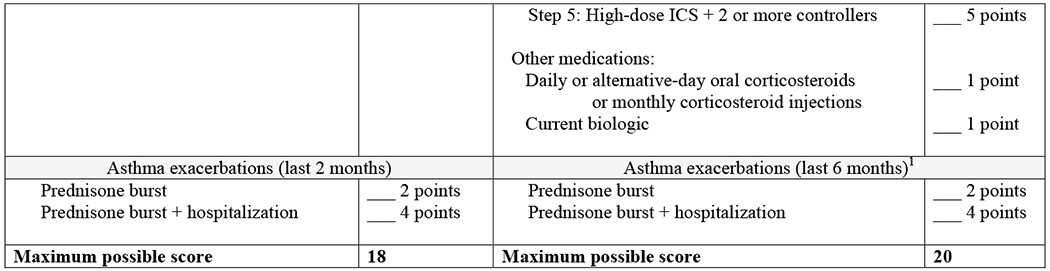 |
ACT = Asthma Control Test, CASI = Composite Asthma Severity Index, ICS = Inhaled corticosteroid, LABA = Long-acting-beta agonist, LTRA = leukotriene receptor antagonist
For the baseline SARP visit, exacerbations were assessed over the preceding 12 months. Follow-up SARP visits (at 12, 24 and 36 months) assessed exacerbations over the preceding 6 months
Scale properties of the Asthma Severity Scoring System
Internal consistency of the ASSESS tool was evaluated with Cronbach’s alpha. Test-retest reliability was evaluated with intraclass correlation using two-way random models with the following format:
which is the preferred approach for evaluation of rater-based clinical assessments that are designed for routine use by any clinician.12 Given that there is no available gold-standard measure for asthma severity in adults, validity could not be determined with traditional approaches. Instead, baseline ASSESS scores were compared in participants treated with ICS who reported 3 or more exacerbations in the previous 12 months, and participants with a hospitalization or intensive care unit admission in the previous year using t-tests. ASSESS scores were also compared in participants who did and did not satisfy the modified dichotomous ERS/ATS definition of severe asthma using Point-Biserial correlation analysis.
Responsiveness of the Asthma Severity Scoring System.
Changes in ASSESS scores were compared to changes in Asthma Control Test (ACT) score, absolute change in FEV1 percent predicted value (calculated as FEV1 % predictedfollow-up visit - FEV1 % predictedprior visit), and the change in the number of asthma controller medications using Pearson correlational analyses. Responsiveness of the ASSESS tool to changes in the Asthma Quality of Life Questionnaire (AQLQ) total score and individual AQLQ domains (symptoms, activities, emotions, environment) was assessed using Pearson correlational analyses. For comparison purposes, the ability of the modified dichotomous ERS/ATS definition of severe asthma to discriminate these same outcomes was also assessed with t-tests.
Mean changes and Minimal Important Difference.
Mean changes in ASSESS scores were further calculated for groups of participants meeting pre-specified cut-points as follows: 1) ACT score, 3 point increase13; 2) FEV1 absolute change, 10% increase;7, 14, 15 3) controller medications, 2 medication decrease; and 4) AQLQ, 0.5 point increase.16 Mean differences between groups were compared with t-tests.
The Minimal Important Difference (MID) of the ASSESS tool was determined at baseline and 12, 24 and 36 months with a distribution-based approach, which utilizes the statistical properties of the scale.17 Data from each corresponding time point were used to determine the standard deviation (SD) and standard error of measurement (SEM). The MID was defined by both 0.5*SD18 and 1*SEM.19 The SEM was calculated as follows:
Where reliability corresponds to the Cronbach α value. To aid in clinical interpretation of MID results, receiver operating characteristic (ROC) analyses were performed with one-year differences in ASSESS scores as the predictor and outcomes of asthma improvement (≥1 fewer exacerbations, ≥1 fewer controller medications, ≥0.5 point increase in AQLQ16) and asthma worsening (≥1 more exacerbation, ≥1 more controller medication, ≥0.5 point decrease in AQLQ16, hospitalization). Analyses were performed in a merged dataset of repeated measures from 12, 24 and 36 months (N=1686 observations).
External replication.
External replication was performed on a sample of 100 participants age ≥12 years enrolled in phase 1 and 2 of SARP program between 2003 and 2010 with baseline and 6 month follow-up data. None of these participants were enrolled in SARP3. Methods for participant characterization in the SARP 1 and 2 program have been published previously.20
Utility for detection of treatment response.
Utility of the ASSESS tool in detecting treatment responses was assessed in participants in SARP3 who underwent corticosteroid response testing with intramuscular triamcinolone. Changes in Asthma Control Questionnaire (ACQ) scores and FEV1 % predicted values before and after triamcinolone receipt were compared in participants with a ≥2 point improvement (i.e., decrease) on the ASSESS tool versus participants with ≤2 point improvement. Exploratory analyses also compared ASSESS scores in participants in whom biologic therapy was initiated or discontinued.
Data analysis.
Data were analyzed with IBM SPSS Statistics (Version 24.0). For all analyses, an alpha level of 0.05 was considered the threshold for statistical significance. Given the exploratory nature of the analyses, no adjustments were made for multiple comparisons.
Results
Five hundred ninety eight participants were initially selected for inclusion; however, 562 participants provided at least 12 months of follow up data and were included in the analysis. Features of these participants are shown in Table 2.
Table 2.
Features of the participants, shown as mean ± SEM or number of participants (%). Groups are not significantly different.
| Feature | N = 562 |
|---|---|
| Age (years) | 44.1 ± 0.7 |
| Age < 18 years | 65 (11.6) |
| Age > 18 years | 497 (88.4) |
| Asthma duration (years) | 28.5 ± 0.7 |
| Male | 202 (35.9) |
| Hispanic ethnicity | 24 (4.3) |
| Race | |
| White | 350 (62.3) |
| Black | 142 (25.3) |
| More than one race | 49 (8.7) |
| Other | 21 (3.7) |
| Severe asthma (modified dichotomous ERS/ATS definition) | 328 (58.4) |
| Highest household educational attainment | |
| Did not complete high school | 10 (1.8) |
| High school diploma | 57 (10.1) |
| Some college or technical training | 115 (20.5) |
| Associate degree | 87 (15.5) |
| Bachelor’s degree | 289 (51.4) |
| Decline to answer | 4 (0.7) |
| Highest annual household income | |
| Less than $25,000 | 104 (18.5) |
| $25,000 to $49,999 | 114 (20.3) |
| $50,000 to $99,999 | 159 (28.3) |
| $100,000 or more | 109 (19.4) |
| Decline to answer | 76 (13.5) |
| Saw an asthma specialist (previous year)1 | 306 (54.4) |
| Asthma healthcare utilization (previous year) | |
| Unscheduled physician visit | 250 (44.5) |
| Emergency department | 143 (25.4) |
| Hospitalization | 70 (12.5) |
| Intubation for asthma (ever in lifetime) | 35 (6.2) |
| Daily asthma medications | |
| Inhaled corticosteroid | 489 (87.0) |
| Long-acting beta-agonist | 439 (78.1) |
| Long-acting anti-muscarinic | 52 (9.3) |
| Leukotriene receptor antagonist | 226 (40.2) |
| Theophylline | 23 (4.1) |
| Systemic corticosteroids | 91 (16.2) |
| Biologic | 43 (7.7) |
| Asthma Control Test Score | 17.2 ± 0.2 |
| 23-25 | 66 (11.8) |
| 20-22 | 153 (27.3) |
| 17-19 | 116 (20.7) |
| 14-16 | 98 (17.5) |
| 11-13 | 65 (11.6) |
| 8-10 | 42 (7.5) |
| 5-7 | 20 (3.6) |
| Baseline lung function (% predicted value) | |
| FVC | 86.7 ± 0.8 |
| FEV1 | 74.2 ± 0.9 |
Severe asthma (modified dichotomous ERS/ATS definition), n = 236 (72.0%), non-severe asthma, n = 70 (29.9%)
ASSESS score distribution.
In the total sample of SARP3 participants, ASSESS scores ranged from 0 to 20 (mean ± standard deviation, 8.78 ± 3.98; 95% confidence interval (CI) for the mean, 8.45 – 9.11). Responses to the individual items of the ASSESS tool were varied, but some clustering was noted in the pattern of responses (Figure E3). For example, one group of participants with high ASSESS scores had poor asthma control yet normal lung function and few exacerbations, whereas another group of participants with high ASSESS scores had poor asthma control with significant airflow obstruction and frequent exacerbations, consistent with the heterogeneity of asthma previously reported in the SARP3 program.21
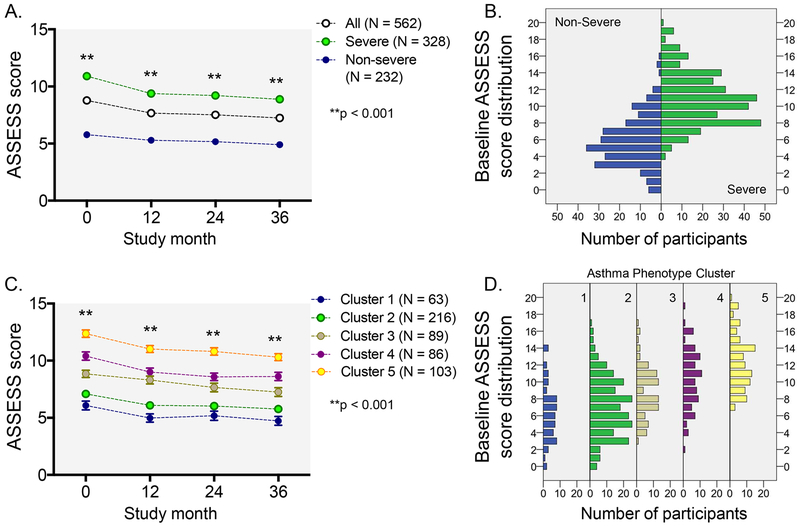
The distribution of ASSESS scores according to the modified dichotomous ERS/ATS definition of severe asthma and SARP Phase 1 and 2 phenotypic cluster assignment (Cluster 1: well controlled early-onset atopic asthma; Cluster 2: early-onset atopic asthma with increased medication requirements; Cluster 3: late-onset, non-atopic obese asthma with moderately reduced lung function and frequent exacerbations; Cluster 4: severe airflow obstruction with bronchodilator reversibility and frequent exacerbations despite multiple controller medications; Cluster 5: severe airflow obstruction with less bronchodilator reversibility and frequent exacerbations despite multiple controller medications) is shown in Figure 1. There were no significant differences in ASSESS scores between adolescents 12-17 years (8.1 ± 3.4) and adults ≥18 years (8.9 ± 4.0).
Figure 1.
Distribution of baseline and longitudinal Asthma Severity Scoring System (ASSESS) scores in all participants, participants according to the modified dichotomous European Respiratory Society/American Thoracic Society definition of severe asthma, and participants according to phenotypic cluster assignment. Data in panels A and B reflect the mean ± SEM.
Reliability measures.
Given the multi-dimensional (versus unidimensional) nature of the ASSESS tool, internal consistency of the item questions was expected to be less than 0.7.22 The Cronbach α value was 0.639 for the entire sample, 0.468 for adolescents 12-17 years, and 0.662 for adults ≥18 years, which reflects an expected lack of concordance between symptoms, lung function, medication use and exacerbations in all asthma patients. However, the intraclass correlation coefficients between baseline and 12 months, 12-24 months, and 24-36 months were 0.764, 0.768, and 0.813 for the entire sample, respectively (Figure E4) and suggested “good” test-retest reliability according to the conventions of Koo et al.12 Intraclass correlation coefficients were also similar between age groups (age 12-17: 0.717, 0.841, 0.732; age ≥18 years: 0.768, 0.766, 0.816). Therefore, further analyses were not stratified by age.
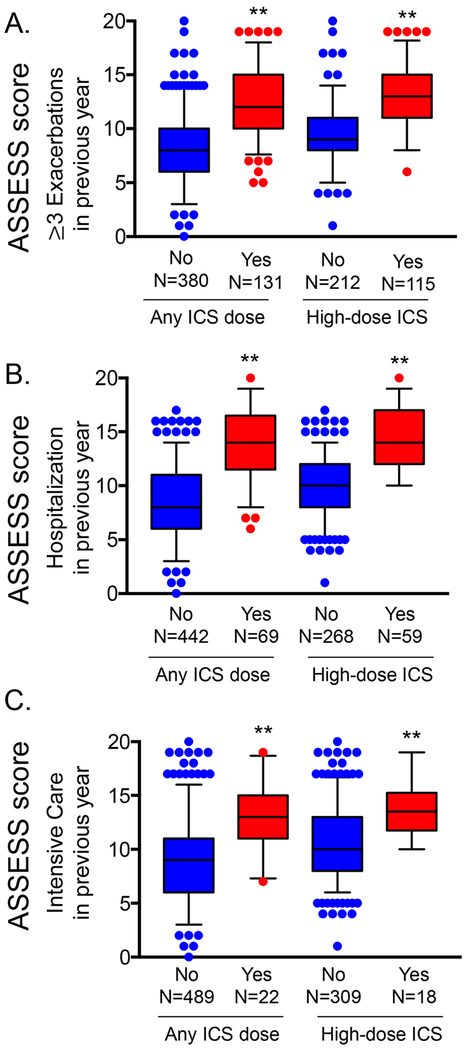
Construct validity.
At baseline, ASSESS scores were higher in participants who reported 3 or more exacerbations, hospitalization or intensive care unit admission for asthma in the previous 12 months (Figure 2). Agreement between ASSESS scores and the modified dichotomous ERS/ATS definition of severe asthma was 0.639 at baseline and 0.635, 0.533, and 0.532 at 12, 24, and 36 months, respectively.
Figure 2.
Asthma Severity Scoring System (ASSESS) scores in participants with 3 or more exacerbations hospitalization, or intensive care unit admission in the previous year. Boxplot whiskers correspond to the 5th and 95th percentiles. **p<0.001
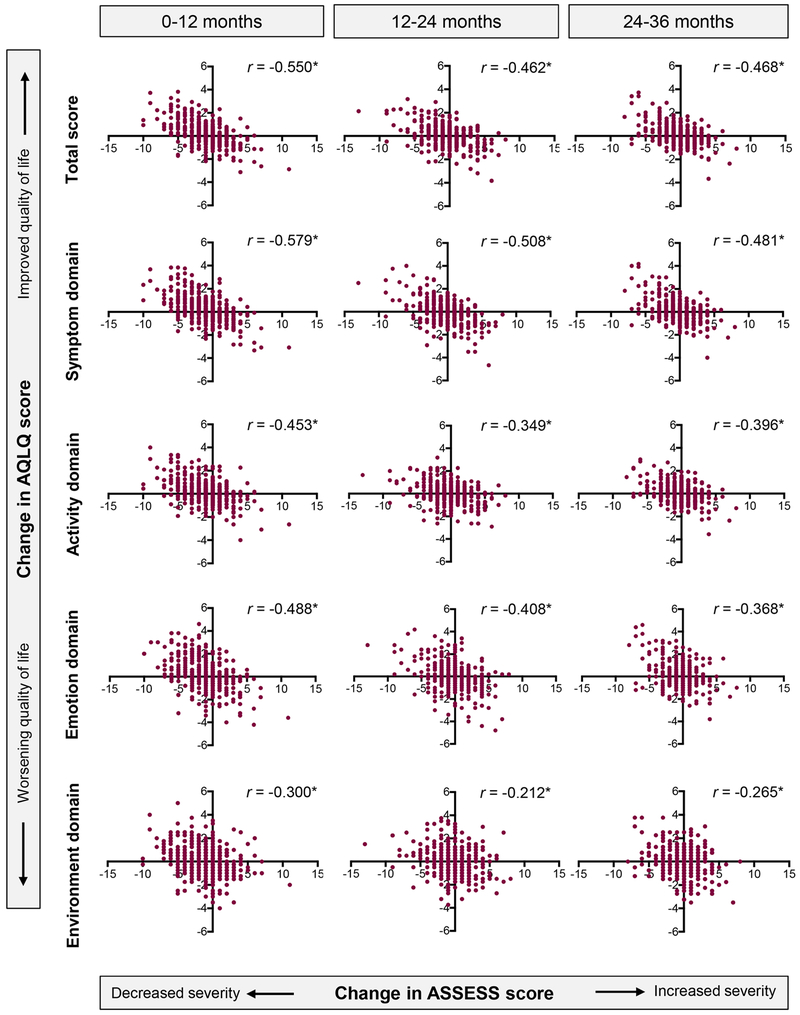
Responsiveness.
By design, the ASSESS tool was sensitive to changes in ACT score, FEV1 % predicted, and the number of asthma controller medications between 0-12 months, 12-24 months, and 24-36 months (Figure E5). The ASSESS tool was also responsive to changes in the AQLQ score (total score and each domain), as shown in Figure 3. In contrast, the modified dichotomous ERS/ATS definition of severe asthma (also assessed at baseline, 12 months and 24 months) did not discriminate changes in these same outcomes (Figure E6).
Figure 3.
Responsiveness of the Asthma Severity Scoring System (ASSESS) score to changes in the Asthma Quality of Life Questionnaire (AQLQ) score between baseline and 12 months, 12 and 24 months, and 24 and 36 months. *p<0.001
Mean differences and Minimal Important Difference.
Mean differences in ASSESS scores between pre-specified participant groups are shown in Table 3 and were approximately 2 overall. Calculation of the ASSESS MID, defined as the smallest difference in the ASSESS score that represents a meaningful change, produced an MID of approximately 2 using a distribution-based approach (for the 0.5*SD calculation, range 1.83-1.96; for the 1*SEM calculation, range 2.39 – 2.56) (Table E4). Clinical interpretation of the calculated ASSESS score MID was further guided by ROC analyses with ASSESS scores as the predictor and outcomes of asthma improvement and worsening reflected by changes in the number of exacerbations, number of controller medications, and AQLQ score and hospitalization occurrence. A MID of 2 had high specificity for outcome detection (80.3 – 85.5% for asthma improvement and 88.0 – 91.4% for asthma worsening), but poor sensitivity (45.8 – 46.5% and 30.0 – 37.3% for improvement and worsening, respectively) (Table E5). A MID of 1 increased sensitivity for asthma improvement (~62%), but only marginally increased sensitivity for asthma worsening (~46%).
Table 3.
Mean difference in ASSESS scores between visits. Negative values indicate a lower ASSESS score at the follow up visit.
| Interval | Asthma outcome | n (%) with outcome | Change in ASSESS score | Mean difference in ASSESS score (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Participants without outcome mean (SD) | Participants with outcome mean (SD) | |||||
| Baseline – 12 months | ACT score, ≥3 point increase | 169 (28.3) | −0.20 ± 2.23 | −3.15 ± 2.34 | −2.95 (−3.36, −2.54) | < 0.001 |
| FEV1, ≥10% absolute increase | 84 (15.1) | −0.77 ± 2.49 | −2.86 ± 2.77 | −2.08 (−2.67, −1.50) | < 0.001 | |
| Medication, decrease of ≥2 | 35 (6.3) | −0.90 ± 2.50 | −3.80 ± 3.11 | −2.90 (−3.77, −2.02) | < 0.001 | |
| AQLQ score, ≥0.5 point increase | 159 (26.6) | −0.44 ± 2.36 | −2.73 ± 2.62 | −2.29 (−2.74, −1.84) | < 0.001 | |
| 12-24 months | ACT score, ≥3 point increase | 111 (18.6) | 0.41 ± 2.12 | −2.46 ± 2.72 | −2.88 (−3.35, −2.40) | < 0.001 |
| FEV1, ≥10% absolute increase | 60 (11.8) | −0.01 ± 2.45 | −1.72 ± 2.79 | −1.71 (−2.38, −1.04) | < 0.001 | |
| Medication, decrease of ≥2 | 5 (1.0) | −0.17 ± 2.52 | −4.00 ± 3.16 | −3.82 (−6.06, −1.60) | 0.001 | |
| AQLQ score, ≥0.5 point increase | 117 (19.6) | 0.21 ± 2.30 | −1.62 ± 2.85 | −1.83 (−2.33, −1.32) | < 0.001 | |
| 24-36 months | ACT score, ≥3 point increase | 97 (16.2) | 0.21 ± 2.00 | −2.25 ± 2.17 | −2.46 (−2.92, −2.00) | < 0.001 |
| FEV1, ≥10% absolute increase | 59 (12.7) | −0.02 ± 2.08 | −2.18 ± 2.60 | −2.16 (−2.75, −1.57) | < 0.001 | |
| Medication, decrease of ≥2 | 11 (2.3) | −0.27 ± 2.23 | −1.45 ± 3.35 | −1.18 (−2.53, 0.17) | 0.087 | |
| AQLQ score, ≥0.5 point increase | 101 (16.9) | 0.12 ± 2.12 | −1.48 ± 2.41 | −1.50 (−1.98, −1.01) | < 0.001 | |
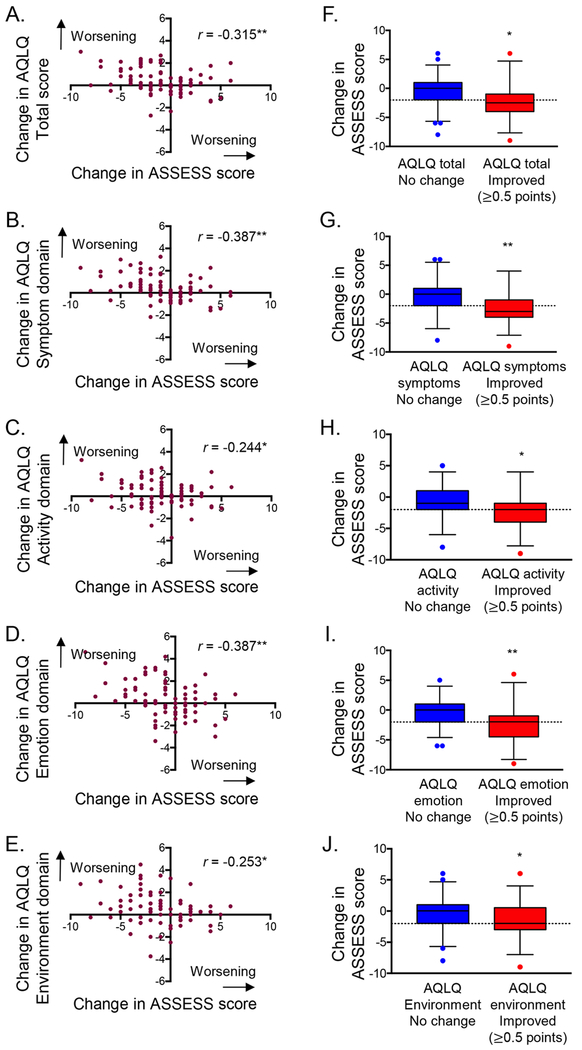
External replication.
Features of the external replication sample are shown in Table E6 and were similar to those of participants in SARP3. Mean ASSESS scores at baseline and the follow-up visit were 8.04 ± 4.61 (95% CI, 6.63 – 8.36) and 7.00 ± 4.42 (95% CI, 5.54 – 7.12). Changes in ASSESS scores were also linearly associated with changes in AQLQ scores (Figure 4A–E). Patients who achieved a clinically meaningful improvement in quality of life (≥0.5 point improvement) also had significant reductions in ASSESS score, although not all participants met the MID of 2 for the ASSESS instrument (Figure 4F–J).
Figure 4.
Associations between changes in Asthma Severity Scoring System (ASSESS) and Asthma Quality of Life Questionnaire (AQLQ) scores in the replication cohort. Figures on the right are stratified by changes in AQLQ scores. Boxplot whiskers and dashed lines correspond to the 5thand 95th percentiles and the minimal important difference of −2 for ASSESS improvement. *p < 0.05, **p<0.01
Utility for detection of treatment response.
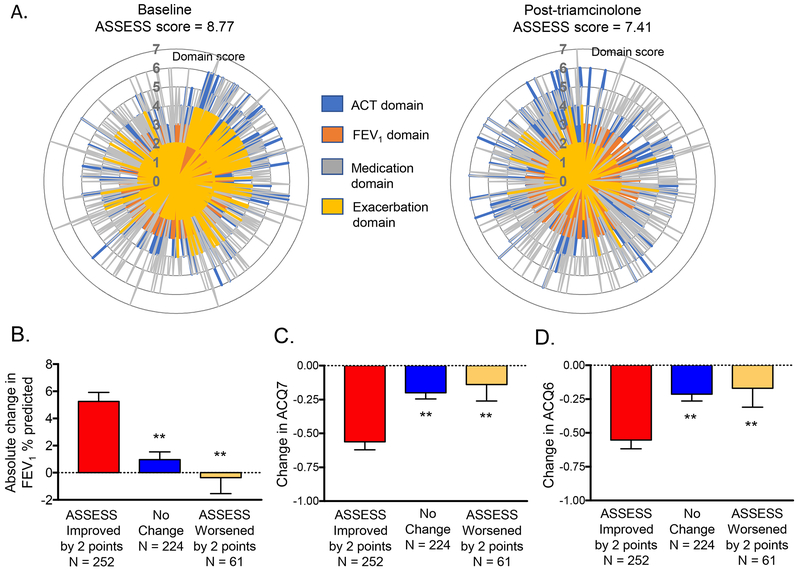
Overall, ASSESS scores were significantly lower after the triamcinolone intervention (p < 0.001, Figure 5A). Participants with ≥2 point improvement (i.e., decrease) in ASSESS scores after triamcinolone also had greater mean improvements in FEV1 % predicted and ACQ scores at 18 ± 3 days after triamcinolone administration (Figure 5B–D). However, these differences were limited to participants with a modified dichotomous ERS/ATS definition of severe asthma (Figure E7, Table E7) and participants assigned to phenotypic clusters 3, 4 and 5 (Figure E8). Further exploratory analyses of patients with severe asthma in whom biologic therapy was initiated (N = 22) also demonstrated higher baseline ASSESS scores than severe patients not treated with biologics and improvement by approximately 2 points (−1.95 ± 3.27) after biologic initiation (Figure E9).
Figure 5.
Radar plots depicting changes in individual domains of the Asthma Severity Scoring System (ASSESS) at baseline and after intramuscular triamcinolone. Spikes correspond to individual participants responses. Panels B-D depict changes (mean ± SEM) in FEVi, ACQ7, and ACQ6 scores after triamcinolone in participants in whom ASSESS scores improved (≥2 point decrease), did not change, and worsened (≥2 point increase). **p<0.01
Discussion
Objective measures of asthma severity are limited, despite the emphasis of asthma severity by treatment guidelines for over a decade.2, 23 The relative explosion of new product development for asthma in recent years now necessitates objective measures for distinguishing patients who might benefit from new treatments such as biologics as well as patients who do not respond to those therapies.24 Advantages of objective versus subjective patient assessments are clearly demonstrated in the asthma literature. For example, other studies of asthma control and asthma functional status have shown that concordance between objective and judgment-based assessments is often poor and may even worsen in patients receiving higher Step-based therapy.25–28 Asthma severity determination is also discordant between physicians in both primary care29 and specialist30 settings. Objective measures such as ASSESS have potential to mitigate the rising costs of asthma severity both in the United States31, 32 and elsewhere33, 34 through comprehensive assessment of treatment responses and ultimately, elimination of unnecessary treatments in patients who do not benefit.
ASSESS was developed in response to the unmet need for a severity scoring system in adolescent and adult patients with asthma.35 Despite the multidimensional nature of the ASSESS tool, it has acceptable measurement properties including test-retest reliability and responsiveness to changes in asthma-related quality of life in heterogeneous asthma populations. Our exploratory analyses also suggest that a reduction (i.e., improvement) of ASSESS scores by at least 2 points may also aid with quantification of response to severe asthma treatments in individual patients and in some phenotypic subgroups. While single outcome variables (such as FEV1 or exacerbation rate) have been used previously in many clinical trials, such narrowly focused analyses may miss the complexity of this disease manifestation and the effects of selected outcome variables on each other. We feel that ASSESS can overcome the limitation of single response variable by offering an integrated numerical score that is sensitive to changes in individual patients. The ASSESS tool is also the first to include biologics and anticholinergics in the assignment of medication treatment step.
Other tools have been proposed for determining asthma severity, but these have limitations in adult asthma populations. The CASI tool was derived from a population of children enrolled in clinical trials11 and the distribution of its components (i.e., lung function measures) differ in adults. The CASI tool also does not address medications that are commonly used in adult asthma populations.11 A separate continuous score of asthma severity developed for adults has also been proposed for epidemiological studies,36 but this score does not adequately capture the constructs of severity as recognized by treatment guidelines and focuses only on symptom frequency and pharmacologic treatment. The coding of the variables in that study (i.e., “yes” or “no” for most symptoms and “none,” “GINA step 1,” or “≥GINA step 2” for medications) also does not distinguish more severe patients with multiple controller medications.36
Nonetheless, there are limitations to our approach. Most importantly, there is no gold standard measure of asthma severity for the purpose of comparison. We instead evaluated performance of the ASSESS score against its own component parts, so it is not surprising that associations were seen. We also were unable to directly compare ASSESS scores to CASI scores. The CASI tool requires that daytime and nighttime symptoms be assessed by self-report using a 2-week recall period prior to the patient encounter, and this information was not available in SARP. However, given the extensive utilization of the ACT tool in clinical practices across the world, our intent was to develop a tool that could utilize information captured by the ACT. An advantage of this approach is that the ACT tool uses a 4-week recall period and collects symptom information as well as information on self-rated asthma control and impact of asthma on functioning. Nonetheless, it is also recognized that despite the 4-week recall window, ACT scores may more strongly reflect symptoms in the immediate 2 weeks.37 We were also limited in our assessment of exacerbation rates at the baseline visit, which impacted tool development, and assessment of treatment responsiveness given the design of the SARP3 study. For example, post-triamcinolone ASSESS scores were calculated from data obtained at the 6-month phone call, with the exception of FEV1, which was obtained at the post-triamcinolone follow-up visit 18 ± 3 days after triamcinolone administration. This long interval between triamcinolone administration and follow-up may not have been optimal for detection of asthma control responses. Indeed, other studies have shown that peak symptom effects with systemic triamcinolone are evident at 7-10 days.38
Although the Cronbach alpha value associated with the ASSESS tool are lower than those associated with other tools, asthma “severity” is not a unidimensional construct and therefore struct cut-points of 0.70 for “acceptability” are not appropriate. Indeed, Brunner et al.22 argue that is worth re-thinking recommended benchmark values for Cronbach alpha in the context of multi-dimensional measures, because scale scores below these values may still contain valuable information and may still have predictive power. That fact that the Cronbach alpha was less than 0.7 was therefore expected and reflects a lack of concordance between symptoms, lung function, medication use and exacerbations in all patients.
The ASSESS score also does not take into account medication adherence and could be limited if prescribing practices are not aligned with treatment recommendations. Indeed, over-prescription of systemic corticosteroids and under-prescription (or under-utilization) of ICS and other asthma controller medications have been reported in the literature and may be particularly problematic in certain patient populations.39–41 The ASSESS tool also focuses on current medications and does not account for treatment duration or the timing of medication initiation (for example, the previous week or 12 months earlier). Generalization to other asthma populations is also cautioned since the ethnic distribution of the SARP3 population was limited and participant recruitment occurred predominately at large academic medical centers across the United States.
The lack of a gold standard measure of asthma severity also poses a challenge for calculation of the MID of the ASSESS tool. The two broad methods of estimating MID, the distribution method and the anchor-based methods, are conceptually different.17 Whereas the distribution method uses the statistical properties of the score distribution, the anchor-based method uses a direct response from the patient on another tool that measures the same construct to evaluate the meaning of a particular degree of change.17 Anchor-based methods often yield more conservative estimates of MID compared with the distribution method42 and thus the clinical meaningfulness of our calculated MID should be interpreted with caution until further studies are performed. We did compare changes in ASSESS scores to changes in patient-reported quality of life, and found the ASSESS tool to be responsive in this regard. Patients achieving a MID of 0.5 point improvement16 on the AQLQ instrument also had improvement (i.e., decrease) of ~2 points on the ASSESS tool, which was similar to our calculated MID value of ~2. The specificity of a 2-point MID was also good, but the sensitivity for outcome detection (either asthma improvement or asthma worsening) was poor. Although a separate analysis of the CASI tool found that a MID of 1 point or greater may be appropriate for future studies,43 a one-point change in ASSESS could occur spuriously (for example, from a 1-point increase in FEV1 from 69 to 70% predicted, or a 1-point increase in ACT score from 10 to 11) and have little clinical meaningfulness. Recognizing that asthma severity is a multi-dimensional construct and not a unidimensional measure, an MID of 2 for ASSESS is also more likely to capture changes in more than one severity dimension.
Despite these limitations, there are a number of strengths. A major strength of the present study is the comprehensive characterization of enrolled participants and enrichment of the study population with severe asthma as defined by ERS/ATS criteria, which has a prevalence rate of less than 5% in the entire asthma population.44 Another strength is the availability of longitudinal data of the majority of the participants, allowing for testing this tool in the same group of subjects over time. The multi-center design of SARP is another strength since asthma features and related outcomes can be subject to wide geographic variability.45, 46 The SARP study also utilized crude measures of socioeconomic status such as education and income and had adequate representation across socioeconomic brackets.
In conclusion, the ASSESS tool, which assesses 4 domains of asthma severity, has acceptable measurement properties and is responsive to changes in asthma quality of life with a MID of approximately 2. ASSESS explains significant variance in selected asthma outcomes and may be useful in epidemiologic and research studies to quantify treatment response in individual patients and in phenotypic subgroups. However, additional validation studies are needed. We caution against the use of ASSESS in clinical practice until such studies are undertaken, to avoid inappropriate changes in asthma treatment.
Supplementary Material
Clinical implications.
The Asthma Severity Scoring System (ASSESS) incorporates four asthma domains and may be useful in research settings for the quantification of treatment response in individual patients and in phenotypic groups.
Acknowledgments
The authors would like to acknowledge Dr. William W. Busse for his input regarding the tool development and for his review of the manuscript.
Supported by NHLBI to the Severe Asthma Research Program (SARP):
U10 HL109086
U10 HL109146
U10 HL109152
U10 HL109164
U10 HL109168
U10 HL109172
U10 HL109250
U10 HL109257
In addition, this program is supported through the following National Institutes of Health National Center for Advancing Translational Sciences awards:
UL1 TR001420 (Wake Forest University)
UL1 TR000427 (University of Wisconsin)
UL1 TR001102 (Harvard University)
UL1 TR002378 (Emory University)
Disclosures:
Anne M. Fitzpatrick:
Nothing to disclose.
Stanley J. Szefler:
Dr. Szefler reports consultancy fees from Merck, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Aerocrine, Novartis, Astra Zeneca, Daiichi Sankyo, Roche, Sanofi, Regeneron and Teva; grants from GlaxoSmithKline, outside the submitted work.
David T. Mauger:
Dr. Mauger reports non-financial support from Merck, non-financial support from Boerhinger-Ingleim, non-financial support from GSK, non-financial support from TEVA, non-financial support from Vifor, outside the submitted work.
Brenda R. Phillips:
Nothing to disclose.
Loren C. Denlinger:
Dr. Denlinger reports personal fees from AstraZeneca, personal fees from Sanofi, personal fees from GSK, outside the submitted work.
Wendy C. Moore:
Dr. Moore reports consultancy fees from AstraZeneca, Sanofi, and GlaxoSmithKline; and is the principal investigator in multicenter clinical trials with sponsors Astrazeneca, GSK, Pearl Therapeutics, and Sanofi.
Ronald L. Sorkness:
Nothing to disclose.
Sally E. Wenzel:
Dr. Wenzel reports grants and personal fees from AstraZeneca , grants from Beohringer-Ingelheim, grants from GSK, grants from Novartis, grants and personal fees from Sanofi , personal fees from Pieris , personal fees from Up to Date, outside the submitted work; In addition, Dr. Wenzel has a patent null pending.
Peter J. Gergen:
Nothing to disclose.
Eugene R. Bleecker:
Dr. Bleecker reports other from ERB has undertaken clinical trials through his employer, Wake Forest School of Medicine and University of Arizona, for AstraZeneca, MedImmune, Boehringer Ingelheim, Genentech, Johnson and Johnson (Janssen), Novartis, Regeneron, and Sanofi Genzyme, personal fees from ERB has also served as a paid consultant for AztraZeneca, MedImmune, Boehringer Ingelheim, Glaxo Smith Kline, Novartis, Regeneron, and Sanofi Genzyme, outside the submitted work.
Mario Castro:
Dr. Castro reports personal fees from Astra-Zeneca, Aviragen, Boehringer-Ingelheim, Boston Scientific, Elsevier, Genentech, 4D Pharma, Mallinckrodt, Neutronic, Nuvaira, Teva, Theravance and VIDA; grants from Boehringer-Ingelheim, Chiesi, Genentech, Novartis, Sanofi-Aventis, Vectura; all outside the submitted work.
Serpil C. Erzurum:
Dr. Erzurum reports serving as the Chair of the American Board of Internal Medicine Pulmonary Disease Board, outside the submitted work.
John V. Fahy:
Dr. Fahy reports consultancy fees from Boehringer Ingelheim, Pieris, Entrinsic Health Solutions, Sanofi Genzyme; and is a named inventor on three patents, outside the submitted work.
Benjamin M. Gaston:
Dr. Gaston reports minority ownership in Respiratory Research, Inc., and intellectual property regarding the treatment of severe asthma.
Elliot Israel:
Dr. Israel reports personal fees from AstraZeneca, personal fees from Novartis, personal fees from Philips Respironics, personal fees from Regeneron Pharmaceuticals, personal fees and other from TEVA Specialty Pharmaceuticals, grants from Genentech, non-financial support from Boehringer Ingelheim, non-financial support from GlaxoSmithKline, non-financial support from Merck, non-financial support from Sunovion, non-financial support from TEVA, grants from Sanofi, personal fees from Bird Rock Bio, personal fees from Nuvelution Pharmaceuticals, personal fees from Vitaeris, Inc, grants from Boehringer Ingelheim, non-financial support from TEVA Specialty Pharmaceuticals, personal fees from Sanofi Genzyme, personal fees from Merck, personal fees from Entrinsic Health Solutions, personal fees from GlaxoSmithKline, other from Vorso Corp., personal fees from Pneuma Respiratory , personal fees from 4D Pharma, outside the submitted work.
Bruce D. Levy:
Nothing to disclose.
Deborah A. Meyers:
Nothing to disclose.
W. Gerald Teague:
Dr. Teague reports salary support from the University of Virginia Ivy Foundation (Endowed Chair); Grant support from Panera Bread, TEVA Respiratory, Astra Zeneca and Sanofi/Regeneron; Advisory Boards for Sanofi/Regeneron, TEVA Respiratory, GSK, Genentech, Aviragen; Speaker Bureaus with personal fees from Genentech/Novartis, TEVA Respiratory (QVAR); and Writing committees for the American College of Allergy and Immunology, outside the submitted work.
Kristie R. Ross:
Dr. Ross reports grants from National Institutes of Health, during the conduct of the study; grants from Ohio Department of Jobs and Family Services, non-financial support from Sunovion, non-financial support from Merck, non-financial support from BI, grants and non-financial support from TEVA, non-financial support from GSK, grants from Astra Zeneca, grants from Roche, outside the submitted work.
Leonard B. Bacharier:
Dr. Bacharier reports personal fees from GlaxoSmithKline, personal fees from Genentech/Novartis, personal fees from Merck, personal fees from DBV Technologies, personal fees from Teva, personal fees from Boehringer Ingelheim, personal fees from Sanofi/Regeneron, personal fees from Vectura, personal fees from Circassia, personal fees from AstraZeneca, outside the submitted work.
Ngoc Ly:
Dr. Ly reports grants from Vertex 2017, personal fees from Gilead 2017, outside the submitted work.
Wanda Phipatanakul:
Nothing to disclose.
Kristie R. Ross:
Nothing to disclose.
Joe Zein:
Dr. Zein reports research support from the Cleveland Clinic.
Nizar N. Jarjour:
Dr. Jarjour received honorarium from Astra Zeneca and Boehringer Ingelheim for consultation unrelated to the submitted work. Dr. Jarjour also served on the ABIM Pulmonary Disease Test Committee, outside the submitted work.
Abbreviations
- ACQ
Asthma Control Questionnaire
- ACT
Asthma Control Test
- ASSESS
Asthma Severity Scoring System
- ATS
American Thoracic Society
- AQLQ
Asthma Quality of Life Questionnaire
- CASI
Composite Asthma Severity Index
- ERS
European Respiratory Society
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- GINA
Global Initiative for Asthma
- ICS
Inhaled corticosteroid
- LABA
Long-acting beta-agonist
- MID
Minimally important difference
- ROC
Receiver operating curve
- SARP
Severe Asthma Research Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.Gov registration number:
References
- 1.Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, et al. A new perspective on concepts of asthma severity and control. Eur Respir J 2008; 32:545–54. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2018. Available from www.ginasthma.org Last accessed November 1, 2018.
- 3.Newby C, Heaney LG, Menzies-Gow A, Niven RM, Mansur A, Bucknall C, et al. Statistical cluster analysis of the British Thoracic Society Severe refractory Asthma Registry: clinical outcomes and phenotype stability. PLoS One 2014; 9:e102987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silkoff PE, Laviolette M, Singh D, FitzGerald JM, Kelsen S, Backer V, et al. Longitudinal stability of asthma characteristics and biomarkers from the Airways Disease Endotyping for Personalized Therapeutics (ADEPT) study. Respir Res 2016; 17:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaihra T, Walsh CJ, Ahmed S, Fugere C, Hamid QA, Olivenstein R, et al. Phenotyping of difficult asthma using longitudinal physiological and biomarker measurements reveals significant differences in stability between clusters. BMC Pulm Med 2016; 16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline Features of the Severe Asthma Research Program (SARP III) Cohort: Differences with Age. J Allergy Clin Immunol Pract 2018; 6:545–54 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, et al. Effects of Age and Disease Severity on Systemic Corticosteroid Responses in Asthma. Am J Respir Crit Care Med 2017; 195:1439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA Jr., Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol 2012; 129:S34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43:343–73. [DOI] [PubMed] [Google Scholar]
- 10.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010; 181:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wildfire JJ, Gergen PJ, Sorkness CA, Mitchell HE, Calatroni A, Kattan M, et al. Development and validation of the Composite Asthma Severity Index--an outcome measure for use in children and adolescents. J Allergy Clin Immunol 2012; 129:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016; 15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol 2009; 124:719–23 e1. [DOI] [PubMed] [Google Scholar]
- 14.Brand PL, Quanjer PH, Postma DS, Kerstjens HA, Koeter GH, Dekhuijzen PN, et al. Interpretation of bronchodilator response in patients with obstructive airways disease. The Dutch Chronic Non-Specific Lung Disease (CNSLD) Study Group. Thorax 1992; 47:429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward H, Cooper BG, Miller MR. Improved criterion for assessing lung function reversibility. Chest 2015; 148:877–86. [DOI] [PubMed] [Google Scholar]
- 16.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol 1994; 47:81–7. [DOI] [PubMed] [Google Scholar]
- 17.Norman GR, Sridhar FG, Guyatt GH, Walter SD. Relation of distribution- and anchor-based approaches in interpretation of changes in health-related quality of life. Med Care 2001; 39:1039–47. [DOI] [PubMed] [Google Scholar]
- 18.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003; 41:582–92. [DOI] [PubMed] [Google Scholar]
- 19.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999; 52:861–73. [DOI] [PubMed] [Google Scholar]
- 20.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am J Respir Crit Care Med 2017; 195:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunner M, SUB H-M . Analyzing the reliability of multidimensional measures: An example from intelligence research. Educ Psychol Meas 2005; 65:227–40. [Google Scholar]
- 23.National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007; 120:S94–138. [DOI] [PubMed] [Google Scholar]
- 24.Tabatabaian F, Ledford DK, Casale TB. Biologic and New Therapies in Asthma. Immunol Allergy Clin North Am 2017; 37:329–43. [DOI] [PubMed] [Google Scholar]
- 25.Crespo-Lessmann A, Plaza V, Gonzalez-Barcala FJ, Fernandez-Sanchez T, Sastre J. Concordance of opinions between patients and physicians and their relationship with symptomatic control and future risk in patients with moderate-severe asthma. BMJ Open Respir Res 2017; 4:e000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urrutia I, Plaza V, Pascual S, Cisneros C, Entrenas LM, Luengo MT, et al. Asthma control and concordance of opinions between patients and pulmonologists. J Asthma 2013; 50:877–83. [DOI] [PubMed] [Google Scholar]
- 27.Menzies-Gow A, Chiu G. Perceptions of asthma control in the United Kingdom: a crosssectional study comparing patient and healthcare professionals’ perceptions of asthma control with validated ACT scores. NPJ Prim Care Respir Med 2017; 27:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juniper EF, Chauhan A, Neville E, Chatterjee A, Svensson K, Mork AC, et al. Clinicians tend to overestimate improvements in asthma control: an unexpected observation. Prim Care Respir J 2004; 13:181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillis RME, van Litsenburg W, van Balkom RH, Muris JW, Smeenk FW. The contribution of an asthma diagnostic consultation service in obtaining an accurate asthma diagnosis for primary care patients: results of a real-life study. NPJ Prim Care Respir Med 2017; 27:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braido F, Baiardini I, Alleri P, Bacci E, Barbetta C, Bellocchia M, et al. Asthma management in a specialist setting: Results of an Italian Respiratory Society survey. Pulm Pharmacol Ther 2017; 44:83–7. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan PW, Campbell JD, Ghushchyan VH, Globe G. Outcomes before and after treatment escalation to Global Initiative for Asthma steps 4 and 5 in severe asthma. Ann Allergy Asthma Immunol 2015; 114:462–9. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan PW, Campbell JD, Ghushchyan VH, Globe G, Lange J, Woolley JM. Characterizing the severe asthma population in the United States: claims-based analysis of three treatment cohorts in the year prior to treatment escalation. J Asthma 2015; 52:669–80. [DOI] [PubMed] [Google Scholar]
- 33.Nordon C, Grimaldi-Bensouda L, Pribil C, Nachbaur G, Amzal B, Thabut G, et al. Clinical and economic burden of severe asthma: A French cohort study. Respir Med 2018; 144:42–9. [DOI] [PubMed] [Google Scholar]
- 34.Barry LE, Sweeney J, O’Neill C, Price D, Heaney LG. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res 2017; 18:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szefler SJ. Asthma across the lifespan: Time for a paradigm shift. J Allergy Clin Immunol 2018; 142:773–80. [DOI] [PubMed] [Google Scholar]
- 36.Calciano L, Corsico AG, Pirina P, Trucco G, Jarvis D, Janson C, et al. Assessment of asthma severity in adults with ever asthma: A continuous score. PLoS One 2017; 12:e0177538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okupa AY, Sorkness CA, Mauger DT, Jackson DJ, Lemanske RF Jr. Daily diaries vs retrospective questionnaires to assess asthma control and therapeutic responses in asthma clinical trials: is participant burden worth the effort? Chest 2013; 143:993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzpatrick AM, Stephenson ST, Brown MR, Nguyen K, Douglas S, Brown LAS. Systemic Corticosteroid Responses in Children with Severe Asthma: Phenotypic and Endotypic Features. J Allergy Clin Immunol Pract 2017; 5:410–9 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farber HJ, Silveira EA, Vicere DR, Kothari VD, Giardino AP. Oral Corticosteroid Prescribing for Children With Asthma in a Medicaid Managed Care Program. Pediatrics 2017; 139. [DOI] [PubMed] [Google Scholar]
- 40.Patel M, Pilcher J, Reddel HK, Qi V, Mackey B, Tranquilino T, et al. Predictors of severe exacerbations, poor asthma control, and beta-agonist overuse for patients with asthma. J Allergy Clin Immunol Pract 2014; 2:751–8. [DOI] [PubMed] [Google Scholar]
- 41.Sweeney J, Patterson CC, O’Neill S, O’Neill C, Plant G, Lynch V, et al. Inappropriate prescribing of combination inhalers in Northern Ireland: retrospective cross-sectional cohort study of prescribing practice in primary care. Prim Care Respir J 2014; 23:74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jayadevappa R, Cook R, Chhatre S. Minimal important difference to infer changes in health-related quality of life-a systematic review. J Clin Epidemiol 2017; 89:188–98. [DOI] [PubMed] [Google Scholar]
- 43.Krouse RZ, Sorkness CA, Wildfire JJ, Calatroni A, Gruchalla R, Hershey GKK, et al. Minimally important differences and risk levels for the Composite Asthma Severity Index. J Allergy Clin Immunol 2017; 139:1052–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Israel E, Reddel HK. Severe and Difficult-to-Treat Asthma in Adults. N Engl J Med 2017; 377:965–76. [DOI] [PubMed] [Google Scholar]
- 45.Malhotra K, Baltrus P, Zhang S, McRoy L, Immergluck LC, Rust G. Geographic and racial variation in asthma prevalence and emergency department use among Medicaid-enrolled children in 14 southern states. J Asthma 2014; 51:913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia E, Serban N, Swann J, Fitzpatrick A. The effect of geographic access on severe health outcomes for pediatric asthma. J Allergy Clin Immunol 2015; 136:610–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.