Abstract
The species from the order Xanthomonadales, which harbors many important plant pathogens and some human pathogens, are currently distinguished primarily on the basis of their branching in the 16S rRNA tree. No molecular or biochemical characteristic is known that is specific for these bacteria. Phylogenetic and comparative analyses were conducted on 26 sequenced Xanthomonadales genomes to delineate their branching order and to identify molecular signatures consisting of conserved signature indels (CSIs) in protein sequences that are specific for these bacteria. In a phylogenetic tree based upon sequences for 28 proteins, Xanthomonadales species formed a strongly supported clade with Rhodanobacter sp. 2APBS1 as its deepest branch. Comparative analyses of protein sequences have identified 13 CSIs in widely distributed proteins such as GlnRS, TypA, MscL, LysRS, LipA, Tgt, LpxA, TolQ, ParE, PolA and TyrB that are unique to all species/strains from this order, but not found in any other bacteria. Fifteen additional CSIs in proteins (viz. CoxD, DnaE, PolA, SucA, AsnB, RecA, PyrG, LigA, MutS and TrmD) are uniquely shared by different Xanthomonadales except Rhodanobacter and in a few cases by Pseudoxanthomonas species, providing further support for the deep branching of these two genera. Five other CSIs are commonly shared by Xanthomonadales and 1–3 species from the orders Chromatiales, Methylococcales and Cardiobacteriales suggesting that these deep branching orders of Gammaproteobacteria might be specifically related. Lastly, 7 CSIs in ValRS, CarB, PyrE, GlyS, RnhB, MinD and X001065 are commonly shared by Xanthomonadales and a limited number of Beta- or Gamma-proteobacteria. Our analysis indicates that these CSIs have likely originated independently and they are not due to lateral gene transfers. The Xanthomonadales-specific CSIs reported here provide novel molecular markers for the identification of these important plant and human pathogens and also as potential targets for development of drugs/agents that specifically target these bacteria.
Introduction
The Xanthomonadales are gram-negative, non-spore forming, catalase-positive, aerobic, rod shape bacteria [1], which are part of the class Gammaproteobacteria [2]. This order is comprised of two families Xanthomonadaceae and Sinobacteraceae that contain 22 and 6 genera, respectively (http://www.bacterio.cict.fr/classifphyla.html#Proteobacteria). The Xylella and Xanthomonas species, which are part of the order Xanthomonadales, cause a wide variety of serious diseases in more than 400 agriculturally important plants. Some of the economically important crops that are affected by species from these two genera include tomato, cabbage, pepper, banana, citrus, rice, grapes, peach, plum, almond, coffee and maple [3]–[9] Additionally, Xylella fastidiosa is responsible for causing leaf scorch disease in many landscape and ornamental plants including oak, elm, mulberry, sycamore, maple and oleander [7], [9]–[11]. The diseases caused by these bacteria lead to major crop losses globally and thus they constitute serious agricultural and economic threat. In addition to these important phytopathogens, the Xanthomonadales also harbors the genus Stenotrophomonas, whose members (viz. S. maltophila) are multidrug resistant opportunistic pathogens, responsible for many hospital-acquired infections in immuno-compromised patients. These latter bacteria are also implicated in respiratory infections in cystic fibrosis patients [12]–[14].
The species from the order Xanthomonadales and its different families/genera are currently distinguished from other bacteria primarily on the basis of their branching in the 16S rRNA trees [1], [4], [15]. There is no biochemical, morphological or physiological characteristics known that are uniquely shared by various species from this order. Although Xanthomonadales are an order within the class Gammaproteobacteria, in phylogenetic trees based upon some genes/proteins sequences, these species are observed to branch with other classes of proteobacteria, particularly the Betaproteobacteria [16]–[20]. However, detailed phylogenetic studies based upon two independent, large datasets of concatenated protein sequences have now established that the species from the order Xanthomonadales are a deep branching clade within the class Gammaproteobacteria [21], [22]. Several recently identified molecular signatures that are uniquely shared by Xanthomonadales and all other Gammaproteobacteria also support the placement of this group within the Gammaproteobacteria [21], [23]. The anomalous branching of Xanthomonadales in some phylogenetic trees possibly results from the deep branching of Xanthomonadales within the Gammaproteobacteria and also in some cases by lateral gene transfers (LGTs). In particular, extensive work by Menck and coworkers indicate that about 25% of the genes in Xanthomonas, which include many genomic islands as well as some genes involved in the biosynthesis of NAD, arginine and cysteine, are acquired by LGTs [16], [24]–[28].
Because Xanthomonadales harbor many major phytopathogens and also some important human pathogens, it is important to understand the evolutionary relationships among these bacteria and identify molecular markers that are specific for either all Xanthomonadales or its different genera. Due to the importance of these bacteria for agriculture and human health, the complete genome sequences for 26 Xanthomonadales species/strains are now available in the NCBI database (see Table 1). In addition, genomes for many other species/strains from this order are currently being sequenced and partial sequence information for them is also available in the databases. These genomes provide valuable resource for discovering molecular and biochemical characteristics that are uniquely shared by these bacteria and which should provide novel means for their identification and also as potential new targets for development of drugs targeting these bacteria. Earlier comparative genomic studies on Xanthomonadales have focused on identifying characteristics that are responsible for the virulence and host specificity of different strains and pathovars of Xanthomonas and Xylella and on understanding the role of LGTs in their genome evolution [3], [4], [4], [7], [8], [11], [29]–[34], [34]–[36]. A recent study on DNA repair proteins also identified four conserved indels that were specific for the available Xanthomonadales species [28]. However, thus far no detailed study has been carried out which is aimed at identifying genetic or molecular characteristics that are uniquely shared by either all Xanthomonadales or its different genera.
Table 1. Sequence Characteristics of Xanthomonadales genomes.
| Organism | GenBank Accession No. | Size(Mbp) | No. of Proteins | % GC content | Reference |
| Stenotrophomonas maltophilia K279a | AM743169.1 | 4.8 | 4386 | 66 | [12] |
| Stenotrophomonas maltophilia R551-3 | CP001111.1 | 4.6 | 4039 | 66 | [12] |
| Stenotrophomonas sp. SKA14 | ACDV00000000 | 4.9 | 4469 | 66 | JCVI * |
| Xanthomonas albilineans GPE PC73 | FP565176.1 | 3.7 | 3114 | 63 | [32] |
| Xanthomonas axonopodis pv. citri str. 306 | AE008923.1 | 5.3 | 4312 | 64 | [56] |
| Xanthomonas axonopodis pv. citrumelo FL 1195 | CP002914.1 | 5.0 | 4181 | 65 | [55] |
| Xanthomonas campestris pv. raphani strain 756C | CP002789.1 | 4.9 | 4520 | 65 | [33] |
| Xanthomonas campestris pv. campestris str. 8004 | CP000050.1 | 5.1 | 4271 | 64 | [30] |
| Xanthomonas campestris pv. campestris str. ATCC 33913 | AE008922.1 | 5.1 | 4179 | 65 | [56] |
| Xanthomonas campestris pv. campestris str. B100 | AM920689.1 | 5.1 | 4466 | 65 | [56] |
| Xanthomonas campestris pv. vesicatoria str. 85-10 | AM039952.1 | 5.4 | 4487 | 64 | [29] |
| Xanthomonas vesicatoria ATCC 35937 | AEQV00000000 | 5.5 | 4927 | 65 | [31] |
| Xanthomonas gardneri ATCC 19865 | AEQX00000000 | 5.5 | 5027 | 65 | [31] |
| Xanthomonas oryzae pv. oryzicola BLS256 | CP003057.1 | 4.8 | 4474 | 64 | [33] |
| Xanthomonas oryzae pv. oryzae KACC10331 | AE013598.1 | 5.0 | 4064 | 63 | [5] |
| Xanthomonas oryzae pv. oryzae MAFF 311018 | AP008229.1 | 5.0 | 4372 | 63 | NIAS* |
| Xanthomonas oryzae pv. oryzae PXO99A | CP000967.1 | 5.2 | 4988 | 63 | [6] |
| Xanthomonas perforans 91-118 | AEQW00000000 | 5.2 | 4637 | 63 | [31] |
| Xylella fastidiosa 9a5c | AE003849.1 | 2.8 | 2766 | 52 | [35] |
| Xylella fastidiosa M23 | CP000941.1 | 2.5 | 2104 | 51 | [10] |
| Xylella fastidiosa M12 | CP001011.1 | 2.6 | 2161 | 51 | [10] |
| Xylella fastidiosa Temecula1 | AE009442.1 | 2.5 | 2034 | 51 | [34] |
| Xylella fastidiosa subsp. fastidiosa GB514 | CP002165 | 2.5 | 2216 | 51 | [57] |
| Pseudoxanthomonas spadix BD-a59 | CP003093.2 | 3.5 | 3149 | 67 | [58] |
| Pseudoxanthomonas suwonensis 11-1 | CP002446.1 | 3.4 | 3070 | 70 | DOE-JGI* |
| Rhodanobacter sp. 2APBS1 | AGIL00000000 | 4.0 | 3800 | 68 | DOE-JGI* |
NIAS = Genome was sequenced by National Institute of Agrobiological Sciences, Japan.
DOE-JGI = Genome was sequenced by DOE Joint Genome Institute USA.
JCVI = Genome was sequenced by J. Craig Venter Institute, USA.
Using genome sequence data, our recent work has focused on identifying Conserved Signature Indels (inserts or deletions) (CSIs) of defined lengths that are present at specific locations in widely distributed proteins and which are uniquely found in particular groups of organisms [37]–[40]. The most parsimonious explanation of these CSIs is that they resulted from highly specific genetic changes that first occurred in a common ancestor of the particular groups of species and were then passed on to various descendants [37], [40], [41]. Further, depending upon the presence or absence of these CSIs in outgroup species, it is possible to infer whether a given CSI is an insert or a deletion and this information can be used to develop rooted phylogenetic relationships independently of phylogenetic trees [21], [37], [42]–[45]. Additionally, the shared presence of some CSIs in unrelated groups of bacteria can also identify possible cases of LGTs [46]. In this work, we report detailed phylogenetic and comparative analyses of protein sequences from Xanthomonadales genomes to identify CSIs that are specific for these organisms. These studies have identified 13 CSIs that are specific for all sequenced Xanthomonadales species and many others CSIs that provide information regarding evolutionary relationships among these bacteria. These molecular signatures provide novel and highly specific means for identification of Xanthomonadales species and for different types of studies on these bacteria. We also report here several CSIs that are commonly shared by Xanthomonadales and either Beta- and/or Alpha-proteobacteria. However, our analysis indicates that the shared presence of these CSIs in Xanthomonadales and these other bacterial groups is due to independent occurrence of similar genetic changes and not due to LGTs.
Methods
Phylogenetic Analyses
Phylogenetic analyses were conducted on a concatenated sequence alignment for 28 conserved and widely distributed proteins that have been widely used for phylogenetic studies [21], [47], [48] and are present in all the Xanthomonadales. These proteins included, alanyl-tRNA synthetase, arginyl-tRNA synthetase, cell division protein FtsY, chaperonin GroEL, dimethyladenosine transferase, DNA gyrase subunit A, DNA gyrase subunit B, DNA polymerase I, DNA-dependent helicase II, elongation factor Tu, histidyl-tRNA synthetase, isoleucyl-tRNA synthetase, methionyl-tRNA synthetase, molecular chaperone DnaK, O-sialoglycoprotein endopeptidase, phenylalanyl-tRNA synthetase subunit alpha, phosphatidate cytidylyltransferase, prolyl-tRNA synthetase, RpoB, RpoC, SecA, SecY, serine hydroxymethyltransferase, seryl-tRNA synthetase, signal recognition particle protein, thioredoxin reductase, tryptophanyl-tRNA synthetase and valyl-tRNA synthetase. For each of these proteins, sequences for all sequenced Xanthomonadales and a number of other Gamma-, Beta- and Alpha-proteobacteria were retrieved by Blastp searches and multiple sequence alignments were created by using the CLUSTAL_X 2.0 [49]. These sequence alignments were concatenated into a single large file and the poorly aligned regions from the alignment were removed using Gblocks 0.91 b program [50]. After removal of poor aligned regions, a total of 14621 aligned positions were present in the final dataset. A neighbor-joining (NJ) tree based on 100 bootstrap replicates was constructed using the JTT matrix-based method [51] in MEGA 5 [52]. A maximum-likelihood tree based upon the same sequence data set was also constructed using the Whelan and Goldman+Freq. model [53] using MEGA5. All positions containing gaps were not considered during these tree constructions.
Identification of Xanthomonadales Specific Conserved Signature Indels (CSIs)
To search for signature sequences in different proteins that are specific for Xanthomonadales or for its subclades, Blastp searches were carried out on each proteins (open reading frame) from the genome of Xylella fastidiosa 9a5c against the NCBI nr database [35]. The results of blast searches were examined for high scoring homologs. For those proteins for whom high scoring homologs (E value <1e−20) were present in Xanthomonadales and several other bacteria, about 10–15 sequences representing different groups were retrieved and multiple sequence alignments were constructed using the CLUSTAL_X 2.0 program [49]. The sequence alignments were visually inspected to identify any conserved indels that were restricted to Xanthomonadales and which were flanked on both sides by at least 5–6 identical/conserved residues in the neighboring 30–50 amino acids [21], [40], [54]. The conserved indels, which in addition to Xanthomonadales were also present in few other species, were also retained. The indels that were not flanked on both sides by conserved regions were not further evaluated as they do not provide useful molecular markers [23], [37], [40]. The species distribution of all indels thus identified (∼150) was further examined by detailed Blastp searches against the nr database (500 top hits) on short sequence segments containing the indels and their flanking conserved regions. Based upon detailed Blast searches, many original indels queries were found to be uninformative for this study due to a variety of reasons including their presence in only a single species/strain, lack of sequence conservation, presence of other confounding indels in the same area in other species, lack of specificity of the indels for any particular group and large variation in their lengths, etc. Hence, such indels were not further studied. However, for different indels those were specific for Xanthomonadales or present in a limited number of other bacteria, sequence information for them were compiled into signature files that are shown here. Due to space considerations, the signature files shown here contain information for only a limited number of species from other bacteria such as Alpha, Beta and Gammaproteobacteria and different strains of the same species are also not shown. However, unless otherwise noted, all of these CSIs are specific for the indicated groups and they are also present in different strains of the Xanthomonadaceae species for which sequence information is available (Table 1).
Results
Phylogenetic Analysis of Genome Sequenced Xanthomonadales
The genome sequences are now available for 26 Xanthomonadales including 15 for Xanthomonas species/strains [5], [6], [29]–[33], [55], [56], 5 for different strains/pathovars of Xylella fastidiosa [10], [34], [35], [57], 3 for Stenotrophomonas species/strains [12], 2 for Pseudoxanthomonas species [58] and for the Rhodanobacter sp. 2APBS1. Some characteristics of these genomes are listed in Table 1. Their genome sizes varied from 2.5 Mb to 5.3 Mb and the xylem-inhabiting bacterium Xylella fastidiosa had the smallest or most reduced genome. Further, in contrast to other Xanthomonadales species/strains whose mol G+C % was in the range of 61–67%, the Xylella strains/pathovars have much lower G+C content. The reduced genome size and the lower G+C mole content of Xylella strains/pathovars have likely resulted from their adaptation to the more stable xylem environment [7].
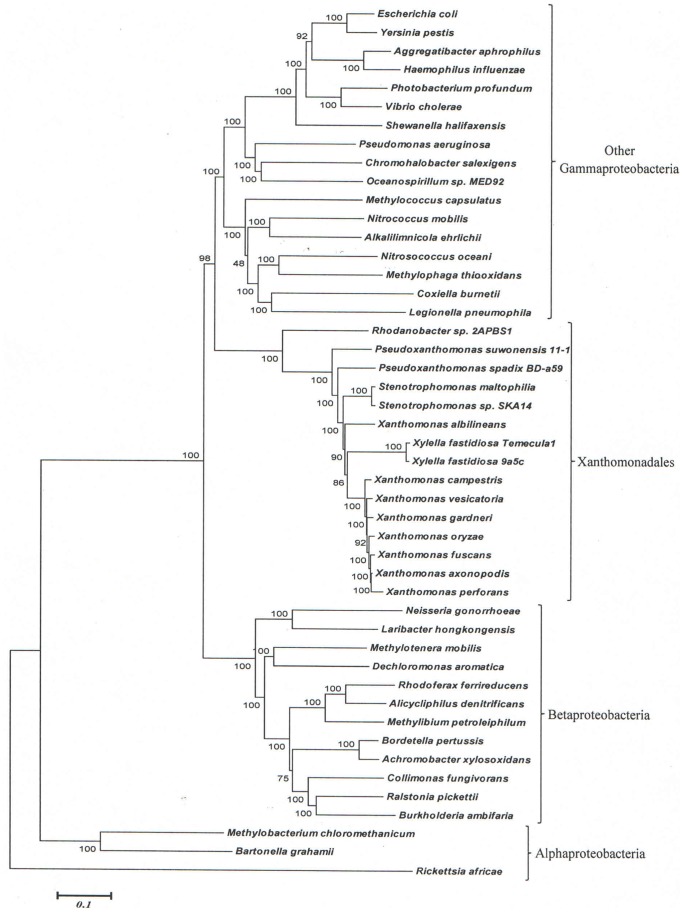
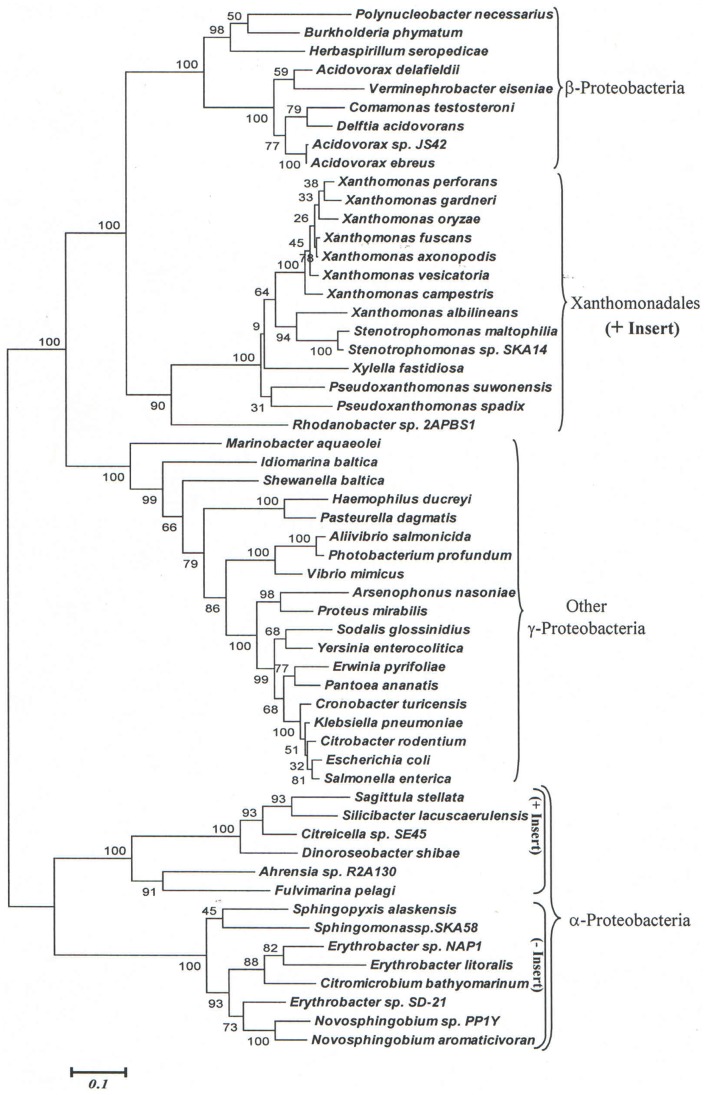
The sequence information from these genomes was also used to examine the evolutionary relationships among the sequenced Xanthomonadales species. Detailed phylogenetic studies on Gammaproteobacteria and other proteobacteria based upon concatenated sequences for different large datasets of protein sequences have been reported previously [19], [21], [22]. In these trees [4], [28], [59], species from the order Xanthomonadales formed a monophyletic clade and one of the deepest branching lineages within the Gammaproteobacteria [21], [22]. Hence, in the present work, phylogenetic trees based upon concatenated sequences were mainly constructed to clarify the branching order of species within the order Xanthomonadales. The dataset employed in this study included sequence information for only a limited number of other proteobacteria. Figure 1 shows a NJ distance tree based upon concatenated protein sequences, which was rooted using sequences from Alphaproteobacteria. The branching order of various Xanthomonadales species in the ML tree (Figure S1) is very similar to that seen in the NJ tree. In both ML and NJ tree, the Xanthomonadales species formed a strongly supported clade branching within the other Gammaproteobacteria. This clade was separated from all other Gammaproteobacteria by a long branch. Similar monophyletic grouping and branching of the Xanthomonadales species within the Gammaproteobacteria have been observed in earlier studies [19], [21], [22]. Among the sequenced Xanthomonadales species, Rhodanobacter was found to be the deepest branching species and it was separated from all other Xanthomonadales by a long branch. Interestingly, the sequenced Xanthomonas species showed polyphyletic branching in the tree, with X. albilineans branching deeply and separately from the other Xanthomonas species (Figure 1 and Figure S1). The tree shown in Figure 1 provides a phylogenetic framework for understanding and interpreting the significance of various CSIs observed in this work.
Figure 1. Phylogenetic tree for Xanthomonadales based on concatenated sequences for 28 conserved proteins.
The tree shown is a NJ distance tree, however, similar branching was observed in the ML tree (Figure S1). The observed bootstrap scores for various nodes are shown on the branch points. The tree was rooted using sequences from Alphaproteobacteria.
Identification of Conserved Signature Indels that are Specific for Xanthomonadales
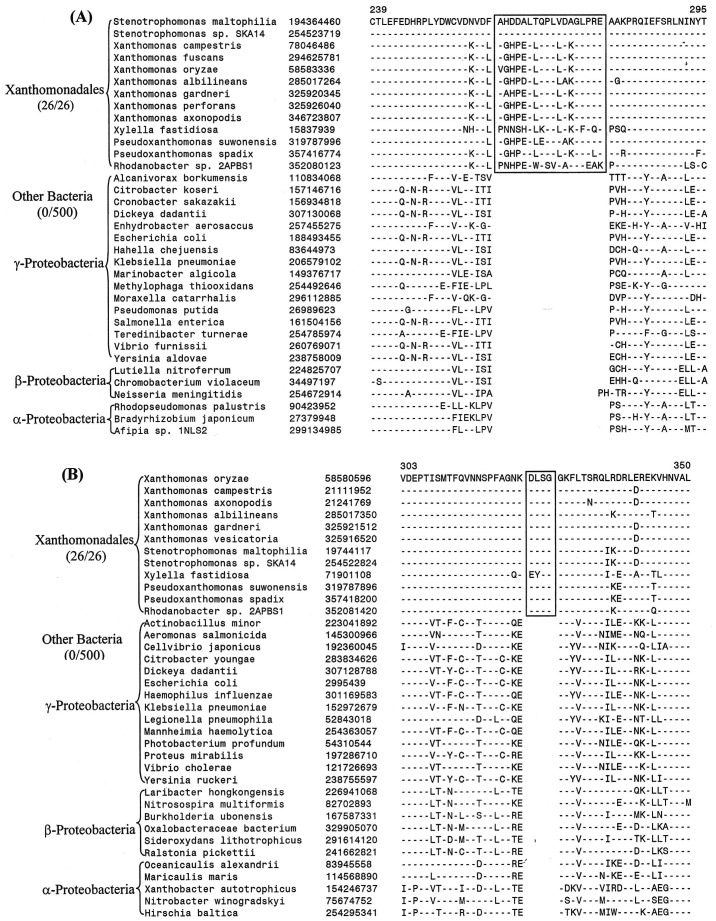
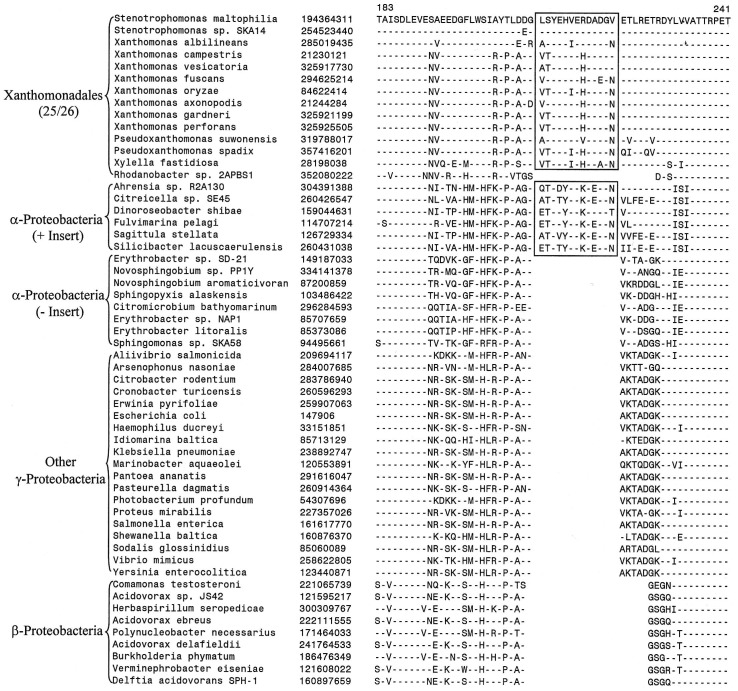
Our work has identified 13 CSIs that are uniquely present in all sequenced Xanthomonadales including the deepest branching Rhodanobacter. Two examples of these CSIs are shown in Figure 2A & B. In the first case (Figure 2A), an 18 aa insert in highly conserved region of the protein glutaminyl-tRNA synthetase, which plays an essential role in protein synthesis by linking glutamine to its cognate tRNA [60]. The large insert in GlnRS is uniquely shared by all available sequences from Xanthomonadales species but not found in any other bacteria (at least the top 500 blast hits). In the other example shown here (Figure 2B), a 4 aa insert in a GTP-binding elongation factor protein (typA) is commonly shared by all sequenced Xanthomonadales, but again it is not found in any other bacteria. Both these CSIs are present in highly conserved regions of the proteins and their sequences are also highly conserved. Because these CSIs are lacking in other bacteria, they constitute inserts in the Xanthomonadales rather than deletions in other bacteria [38]. The sequence information for other CSIs that are uniquely present in all sequenced species/strains of Xanthomonadales is presented in Figures S2–S12 and a summary of their characteristics is provided in Table 2 (first 13 entries). These CSIs include a 7 aa insert in amino acid/peptide transported protein; 5 aa insert in conserved region of the large-conductance mechanosensitive channel protein; a 3 aa insert in LysRS; 2 aa insert in highly conserved region of the protein lipoyl synthase (LipA); 1 aa inserts in the proteins Tgt, LpxA and TolQ; a 13 aa deletion in alpha-2-macroglobulin domain-containing protein and 1 aa deletions in the ParE, PolA and TyrB proteins. Because these CSIs are present in all sequenced Xanthomonadales but not found in any other bacteria, the most likely explanation is that genetic changes responsible for them first occurred in a common ancestor of the Xanthomonadales and then passed on to various descendants by vertical descent.
Figure 2. Examples of conserved signature indels (CSIs) that are specific for the order Xanthomonadales.
Excerpts are shown from the sequence alignments of (A) Glutaminyl t-RNA synthetase and (B) GTP-binding elongation factor proteins showing two CSIs that are uniquely found in various sequenced Xanthomonadales species, but not found in any other bacteria. Information for other CSIs that are specific for the Xanthomonadales is provided in Figures S2–S12 and Table 2. The dashes in these as well as all other alignments show identity with the amino acid on the top line. The Gene bank identification numbers of various sequences are shown in the second column and the numbers on the top indicate the position of this sequence in the species shown on the top line. The sequence information is shown here for only representative species. However, unless otherwise indicated, these CSIs are highly specific for the indicated group of species.
Table 2. Conserved Signatures Indels that are specific for Xanthomonadales.
| Protein Name | Gene Name | GenBankIdentifier | Figure No | Indel Size | Indel Positiona | Exceptionsb |
| Glutaminyl-tRNA synthetase | glnS | 194364460 | Figure 2A | 18 aa ins | 239–295 | None |
| GTP-binding elongation factor protein | typA | 58580596 | Figure. 2B | 4 aa ins | 303–350 | None |
| Amino acid/peptide transporter | – | 71275790 | Figure S2 | 7 aa ins | 164–213 | None |
| Large-conductance mechanosensitive channel | mscL | 294667079 | Figure S3 | 5 aa ins | 40–85 | None |
| Lysyl-tRNA synthetase | lysS | 194365604 | Figure S4 | 3 aa ins | 34–85 | None |
| Lipoyl synthase | lipA | 58583575 | Figure S5 | 2 aa ins | 156–209 | None |
| Queuine tRNA-ribosyltransferase | tgt | 194365393 | Figure S6 | 1 aa ins | 289–339 | None |
| Acyl-(acyl-carrier-protein)–UDP-N-acetyl-glucosamine O-acyltransferase | lpxA | 71275623 | Figure S7 | 1 aa ins | 164–210 | None |
| TolQ protein | tolQ | 21232451 | Figure S8 | 1 aa ins | 177–217 | None |
| Alpha-2-macroglobulin domain-containing protein | – | 194366795 | Figure S9 | 13 aa del | 607–661 | None |
| DNA topoisomerase IV subunit B | parE | 84624476 | Figure S10 | 1 aa del | 282–326 | None |
| DNA polymerase I | polA | 194367713 | Figure S11 | 1 aa del | 28–65 | None |
| Aromatic amino acid aminotransferase | tyrB | 28197970 | Figure S12 | 1 aa del | 306–354 | None |
| Glutaminyl-tRNA synthetase | glnS | 194364460 | Figure 3 | 1 aa del | 77–131 | Methylobacter tundripaludum, Methylomicrobium album BG8,Dichelobacter nodosus |
| DNA polymerase III subunit beta | RpoB | 194363780 | Figure S13 | 1 aa del | 44–81 | Marinomonas sp. MWYL1, Thioalkalivibrio sp. HL-EbGR7 |
| Lipid-A-disaccharide synthase | lpxB | 190573490 | Figure S14 | 2 aa ins | 317–358 | Cardiobacterium hominis, Allochromatium vinosum, Alteromonadales bacterium |
| Carbamoyl phosphate synthase large subunit | carB | 166711938 | Figure S15 | 1 aa ins | 403–457 | Marinobacter sp. ELB17 |
| Putative secreted protein | – | 188992701 | Figure S16 | 1 aa ins | 1285–1318 | Teredinibacter turnerae |
| Aminopeptidase P | pepP | 294627124 | Figure S17 | 1 aa del | 211–246 | Thioalkalivibrio sp. HL-EbGR7,Alkalilimnicola ehrlichii |
The indel position provided indicates the region of the protein containing the CSI.
For details go to respective figures.
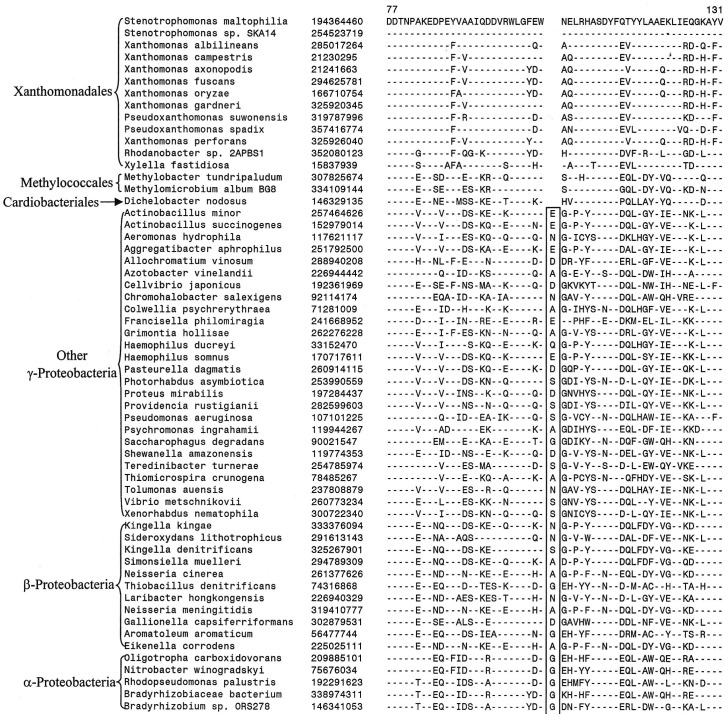
In addition to these CSIs that are uniquely found in all Xanthomonadales, we have also come across 6 other CSIs, where in addition to the Xanthomonadales, the identified CSIs are also present in 1–3 other Gammaproteobacteria. These species are generally from some of the other deep branching orders of Gammaproteobacteria such as Chromatiales, Methylococcales and Cardiobacteriales, which branch in the proximity of Xanthomonadales [21], [22], [28]. One example of such a CSI consisting of a 1 aa deletion in a conserved region of the protein glutaminyl-tRNA synthetase that is commonly shared by various Xanthomonadales and also by a few Methylococcales and Cardiobacteriales species is presented in Figure 3. Sequence information for others CSIs of this kind is presented in Figures S13–S17 and in Table 2 (last six records). Cutino-Jimenez et al. [28]also reported a CSI in Topoisomerase I that was commonly shared by various Xanthomonadales, Methylococcales, Cardiobacteriales, Chromatiales, Legionellales and Thiotrichales. The information provided by these CSIs could prove useful in establishing a specific relationship of the Xanthomonadales to these other deep branching orders of Gammaproteobacteria.
Figure 3. Partial sequence alignment of glutaminyl t-RNA synthetase showing a CSI that is specifically present in various sequenced Xanthomonadales and some other Gammaproteobacteria.
This CSI as well as a few other CSIs identified in this work (see Table 2 and Figures S13–S17) suggest a possible relationship of Xanthomonadales to these deep branching orders of Gammaproteobacteria.
CSIs Supporting the Deeper Branching of Rhodanobacter within the Xanthomonadales
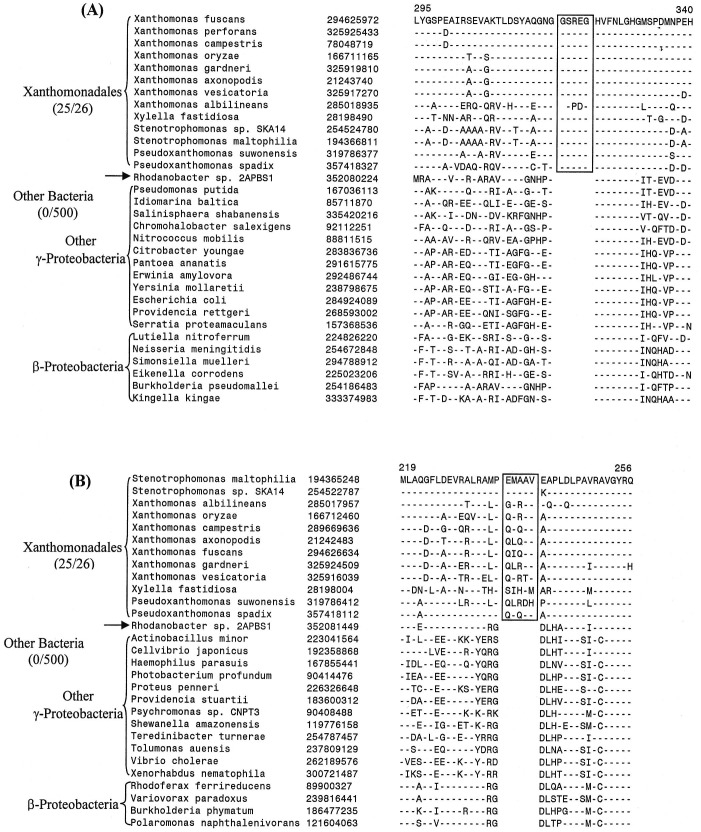
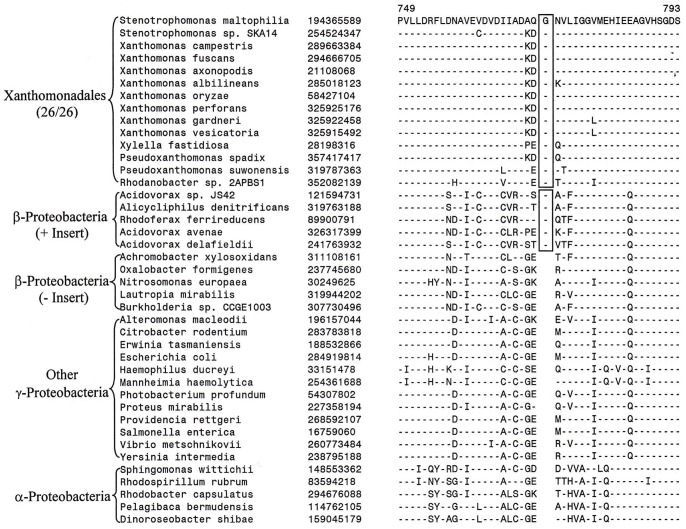
In the phylogenetic tree shown in Figure 1 and Figure S1, Rhodanobacter sp. 2APBS1 exhibited the deepest branching amongst the sequenced Xanthomonadales. During our analyses, we have found 15 CSIs that are uniquely shared by all other Xanthomonadales except Rhodanobacter, supporting the deeper branching of this species in comparison to other Xanthomonadales. Two examples of such CSIs that are uniquely found in different Xanthomonadales, but not in Rhodanobacter are shown in Figure 4A & B. In both these cases 5 aa inserts in highly conserved regions of the proteins uroporphyrinogen decarboxylase (Figure 4A) and in the protein tRNA delta(2)-isopentenylpyrophosphate transferase (Figure 4B) are uniquely shared by different sequenced Xanthomonadales except Rhodanobacter. These CSIs are not present in any other bacteria. A summary of the characteristics of different CSIs showing this type of species distribution pattern is presented in Table 3 and the sequence alignments of the corresponding proteins are provided as Figures S18–S30. The proteins in which these CSIs are found include protoheme IX farnesyltransferase (CoxD), DNA polymerase III alpha subunit (DnaE), DEAD box helicase domain-containing protein, ribose-5-phosphate isomerase A (RpiA), DNA polymerase I (PolA), glucose-6-phosphate 1-dehydrogenase (Zwf1), AspRS, 2-oxoglutarate-dehydrogenase E1 component (SucA), coproporphyrinogen III oxidase (CpoX), and TrmD. In a few of these cases, the CSIs under consideration was also not found in one or both of the Pseudoxanthomonas species, supporting their deeper branching in comparison to other Xanthomonadales genera (viz. Xylella, Xanthomonas and Stenotrophomonas) (Figures S32–S34). In a recent study, Cutino-Jimenez et al. [28] had reported four CSIs in DNA repair proteins that were indicated to be specific for Xanthomonadales. Our analyses of these CSIs, which were also identified in our work, indicate that they are lacking in either Rhodanobacter (4 aa insert in DnaE and 1 aa insert in RecA) or both Rhodanobacter and in P. suwonensis (5 aa insert in MutS and >50 aa insert in LigA) (Figures S29–S31 and S35). The information for these CSIs is also summarized in Table 3. Based upon the species distribution of these CSIs and the branching positions of Rhodanobacter (and Pseudoxanthomonas) in phylogenetic trees, the genetic changes responsible for these CSIs likely occurred in common ancestors of other Xanthomonadales species after the divergence of Rhodanobacter sp. 2APBS1 and also in some cases that of Pseudoxanthomonas species.
Figure 4. Examples of CSIs those are present in various Xanthomonadales species except Rhodanobacter sp. 2APBS1.
Excerpts are shown from the sequence alignments of (A) uroporphyrinogen decarboxylase (HemE) and (B) tRNA delta(2)-isopentenylpyrophosphate transferase (MiaA) proteins showing two conserved signature indels (boxed) that are specifically found in various sequenced Xanthomonadales species, except Rhodanobacter sp. 2APBS1. These CSIs were likely introduced in these genes in a common ancestor of the Xanthomonadales after branching of Rhodanobacter. Information for CSIs in other proteins showing similar species specificities is provided in Figures S18–S30 and Table 3.
Table 3. CSIs that are specific for Xanthomonadales except Rhodanobacter sp. 2APBS1.
| Protein Name | Gene Name | GenBankIdentifier | Figure No | Indel Size | Indel Positiona | Specificity within Xanthomonadales |
| Uroporphyrinogen decarboxylase | hemE | 294625972 | Figure 4A | 5 aa ins | 295–340 | All except Rhodanobacter sp. 2APBS1 |
| tRNA delta(2)-isopentenylpyrophosphate transferase | miaA | 194365248 | Figure 4B | 5 aa ins | 219–256 | All except Rhodanobacter sp. 2APBS1 |
| Protoheme IX farnesyltransferase | coxD | 15837961 | Figure S18 | 4 aa ins | 150–192 | All except Rhodanobacter sp. 2APBS1 |
| DNA polymerase III subunit alpha | dnaE | 21242159 | Figure S19 | 1 aa ins | 583–638 | All except Rhodanobacter sp. 2APBS1 |
| DEAD box helicase domain-containing protein | – | 194364258 | Figure S20 | 1 aa ins | 155–200 | All except Rhodanobacter sp. 2APBS1 |
| Ribose-5-phosphate isomerase A | rpiA | 194367055 | Figure S21 | 1 aa ins | 127–169 | All except Rhodanobacter sp. 2APBS1 |
| DNA polymerase I | polA | 21244827 | Figure S22 | 1 aa ins | 136–180 | All except Rhodanobacter sp. 2APBS1 |
| Aspartyl-tRNA synthetase | aspS | 194366904 | Figure S23 | 4 aa del | 343–391 | All except Rhodanobacter sp. 2APBS1 |
| 2-oxoglutarate-dehydrogenase E1 component | sucA | 194366403 | Figure S24 | 1 aa del | 782–830 | All except Rhodanobacter sp. 2APBS1 |
| Coproporphyrinogen III oxidase | cpoX | 194367710 | Figure S25 | 1 aa del | 166–215 | All except Rhodanobacter sp. 2APBS1 |
| 2-oxoglutarate-dehydrogenase E1 component | sucA | 194366403 | Figure S26 | 1 aa del | 106–164 | All except Rhodanobacter sp. 2APBS1 |
| Asparagine synthase b protein | asnB | 285018780 | Figure S27 | 4–5 aa ins | 404–445 | All except Rhodanobacter sp. 2APBS1 |
| Asparagine synthase b protein | asnB | 194365058 | Figure S28 | 1–2 aa ins | 96–132 | All except Rhodanobacter sp. 2APBS1 |
| DNA polymerase III subunit alpha b | dnaE | 77747494 | Figure S29 | 4 aa ins | 522–576 | All except Rhodanobacter sp. 2APBS1 |
| DNA repair protein RecAb | recA | 15836728 | Figure S30 | 2 aa ins | 172–238 | All except Rhodanobacter sp. 2APBS1 |
| 5′-nucleotidase | – | 21231001 | Figure 5A | 11–13 aa ins | 123–188 | All except Rhodanobacter sp. 2APBS1& Pseudoxanthomonas suwonensis |
| CTP synthetase | pyrG | 194365226 | Figure 5B | 2 aa ins | 253–291 | All except Rhodanobacter sp. 2APBS1 Pseudoxanthomonas suwonensis & Pseudoxanthomonas suwonensis |
| DNA mismatch repair protein MutS b | mutS | 15838317 | Figure S31 | 5 aa ins | 765–806 | All except Rhodanobacter sp. 2APBS1 & Pseudoxanthomonas suwonensis |
| DNA polymerase III subunit alpha | dnaE | 194365029 | Figure S32 | 2 aa del | 65–120 | All except Rhodanobacter sp. 2APBS1 & Pseudoxanthomonas suwonensis |
| tRNA (guanine-N(1)-)-methyltransferase | trmD | 194364933 | Figure S33 | 2 aa ins | 140–200 | All except Rhodanobacter sp. 2APBS1 & Pseudoxanthomonas suwonensis |
| Glucose-6-phosphate 1-dehydrogenase | zwf | 190573773 | Figure S34 | 4 aa del | 290–334 | All except Rhodanobacter sp. 2APBS1 & Pseudoxanthomonas spadix BD-a59 |
| DNA ligase NAD dependent b | ligA | 77747612 | Figure S35 | 57–65 aa ins | 461–583 | All except Rhodanobacter sp. 2APBS1 & Pseudoxanthomonas suwonensis |
The indel position provided indicates the region of the protein containing the CSI.
These CSIs have been previously described [28].
In addition to the CSIs discussed above 4 other proteins contains CSIs of different lengths at the same position, which are uniquely shared by all sequenced species/strains of Xanthomonadales except Rhodanobacter sp. 2APBS1 and in some cases Pseudoxanthomonas. However, these CSIs due to differences in their lengths are also able to distinguish between different genera of Xanthomonadaceae. Two examples of such CSIs are presented in Figure 5. In the first case in the protein 5′-nucleotidase (Figure 5A), which catalyzes the hydrolysis of nucleotides to nucleosides, a 13 aa insert is uniquely shared by all Xanthomonas and Xylella species, whereas the two Stenotrophomonas species have an 11 aa insert in the same position. Because both these CSIs are present at the same position and they are related in sequences, the most likely explanation about their occurrence is that a 13 aa insert was initially introduced in a common ancestor of the Xanthomonas, Xylella and Stenotrophomonas genera and it was followed by a 2 aa deletion in the genus Stenotrophomonas. Alternatively, an 11 aa insert was initially introduced in a common ancestor of these three genera followed by another 2 aa insert in a common ancestor of the Xanthomonas and Xylella genera. Likewise, in a conserved region of the asparagine synthase b protein (AsnB), a 5 aa insert is present in various Xylella, Xanthomonas and Pseudoxanthomonas, whereas the two Stenotrophomonas species have a smaller insert (4 aa) in this position (Figure S27). The AsnB protein also contains another CSI in a different position (see Figure S28), where a 1 aa insert is present in Xylella, Xanthomonas and Pseudoxanthomonas, species, whereas the two Stenotrophomonas species have a 2 aa insert in the same position. In another example of this kind, in the protein CTP synthetase, a 2 aa insert in a conserved region is uniquely shared by various Xylella, Xanthomonas and Stenotrophomonas species/strains, whereas the two Pseudoxanthomonas species contain a 1 aa insert in this position (Figure 5B). These CSIs, in addition to supporting the deeper branching of Rhodanobacter in comparison to other Xanthomonadales, also serve to differentiate Stenotrophomonas and Pseudoxanthomonas species from other genera of Xanthomonadaceae.
Figure 5. Example of CSIs those are able to distinguish two different clades of Xanthomonadales.
Partial sequence alignments are shown of the proteins (A) 5′-nucleotidase and (B) CTP synthetase showing two CSI, which due to their different lengths are able to distinguish between two different clades of Xanthomonadales. In (A), a 13 aa insert is present in all of the Xanthomonas and Xylella species, whereas the two Stenotrophomonas spp. contain an 11 aa insert in this position. Similarly, in (B), all of the Xanthomonas, Xylella and Stenotrophomonas species have a 2 aa insert, whereas the two Pseudoxanthomonas spp. contain a 1 aa insert in this position. Different possibilities to account for these CSIs are discussed in the text.
CSIs that are Commonly Shared by Xanthomonadales and Some Alpha- and Beta-proteobacteria
In addition to the above proteins that contained CSIs, which were highly specific for Xanthomonadales species (or 1–2 closely related species), our analyses have also identified 7 other CSIs, which in addition to various Xanthomonadales are also shared by some Betaproteobacteria and/or Alphaproteobacteria. Two examples of these CSIs are shown in Figures 6 and 7. In the protein valyl-tRNA synthetase, which plays an essential role in protein synthesis, a 13 aa insert in a highly conserved region is present in all sequenced Xanthomonadales, except Rhodanobacter (Figure 6). Interestingly, a very similar CSI is also present in several species belonging to the class Alphaproteobacteria (e.g. Ahrensia sp. R2A130, Labrenzia alexandrii, Rhodobacter capsulatus, Sagittula stellata etc.) whereas other Alphaproteobacteria do not contain this insert. In the other example shown here (Figure 7), in the protein carbamoyl phosphate synthase large subunit (CarB), a 1 aa insert in a conserved region is commonly shared by various Xanthomonadales and a subgroup of Betaproteobacteria (mainly Burkholderiales), but not by any other bacterial groups. The shared presence of similar CSIs by different Xanthomonadales and species from these other classes of proteobacteria could result from a variety of possibilities including lateral transfers of genes for these proteins between these two groups of bacteria or alternatively by independent occurrence of similar genetic changes in these lineages.
Figure 6. Partial sequence alignments of valyl t-RNA synthetase showing a 13 aa insert that is commonly shared by various Xanthomonadales and a subgroup of Alphaproteobacteria.
Other Alpha- and Gamma-proteobacteria do not contain this insert.
Figure 7. Partial sequence alignment of carbamoyl phosphate synthase showing a 1 aa insert that is commonly shared by Xanthomonadales and a subgroup of Betaproteobacteria.
The distinct branching of these two groups in a phylogenetic tree based upon CarB sequence (Figure S36) provides evidence that this shared CSIs is not a result of LGT.
To distinguish between these possibilities, phylogenetic trees for the ValRS and CarB sequences for the same species as shown in Figures 6 and Figure 7 were constructed. In the tree based upon ValRS sequences, which is shown Figure 8, all of the Alphaproteobacteria species (both containing and lacking the insert) formed a strongly supported clade that branched distinctly from the Xanthomonadales. The Xanthomonadales species in this tree branched in between the clades consisting of Betaproteobacteria and the other Gammaproteobacteria, but that is not surprising in view of phylogenetic position within the Gammaproteobacteria. If the shared presence of the CSI in the Xanthomonadales and the CSI-containing Alphaproteobacteria was due to LGTs, then the Alphaproteobacteria containing this CSI should have branched with the Xanthomonadales, which is not observed here. Similarly, in the tree based upon CarB sequences (Figure S36), all of the Betaproteobacteria branched together and no association was observed between the insert containing Betaproteobacteria and the Xanthomonadales. These results do not support the possibility that LGT was responsible for the shared presence of CSIs in these two groups. Instead in the phylogenetic trees shown in Figure 8 and Figure S36, the clades comprising of the inserts containing Alphaproteobacteria or Betaproteobacteria formed distinct subclades within the rest of the Alpha- or Beta-proteobacteria. Thus, it is likely that the genetic changes responsible for these CSI occurred independently in the common ancestors of these subclades of species.
Figure 8. Phylogenetic tree based upon valyl t-RNA synthetase sequences.
The distinct branching of Xanthomonadales and the Alphaproteobacteria containing this insert suggests that the shared presence of this CSIs in these two groups is not due to a LGT.
Besides these two proteins that contained CSIs, which were commonly shared by Xanthomonadales and either some Alpha- or Beta-proteobacteria, five other proteins were identified that contained CSIs showing similar species distributions. These included: two CSIs consisting of 1 aa conserved deletions in a hypothetical protein XOO1065 and the protein orotate phosphoribosyltransferase (PyrE) that are commonly shared by various Xanthomonadales and some Betaproteobacteria (Figures S37 and S38); two CSIs consisting of 1 aa and 2 aa inserts in the proteins putative ribonuclease HII (RnhB) and glycyl-tRNA synthetase subunit beta (GlyS) that are also commonly shared by various Xanthomonadales and some Betaproteobacteria (Figures S39 and S40); a 1 aa deletion in a conserved region in the septum site-determining protein MinD that is commonly shared by Xanthomonadales and some Alpha- and Beta-proteobacteria (Figure S41). The phylogenetic trees based upon the sequences of these proteins are shown in Figures S42 to S46. In all of these trees, the proteobacterial groups which contained similar CSIs as found in the Xanthomonadales did not branch with the Xanthomonadales. These results provide evidence that the CSIs in these other bacterial groups have originated independently and their shared presence is not due to LGTs from Xanthomonadales.
Discussion
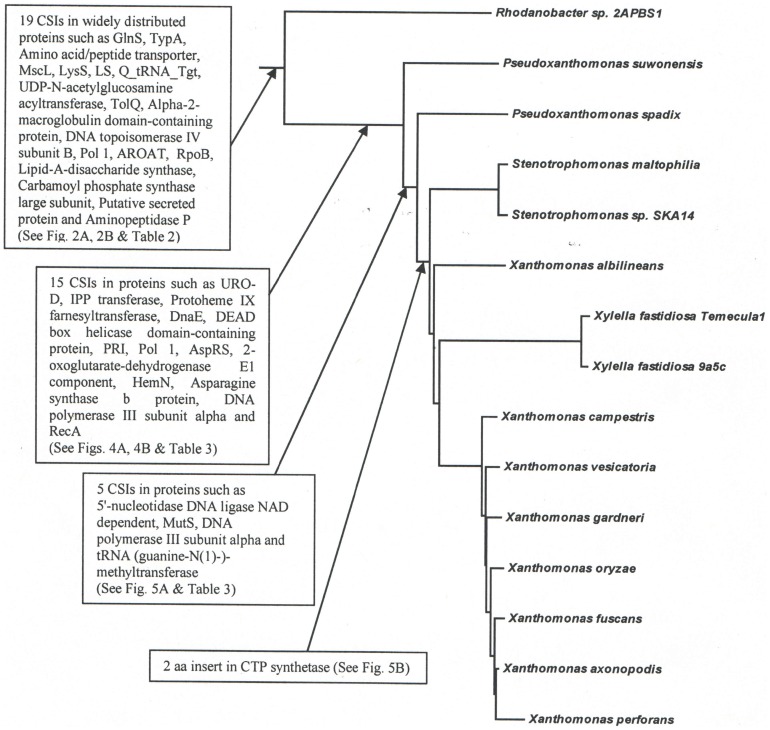
The Xanthomonadales species harbor many major plant pathogens [3], [4], [9] as well as some important human pathogens. However, these bacteria are presently distinguished from other bacteria solely on the basis of their branching in phylogenetic trees (primarily 16S rRNA) and no molecular or biochemical characteristic that is uniquely shared by various species from this group of bacteria is currently known [1]. This paper reports detailed phylogenetic and comparative genomic analyses of sequenced Xanthomonadales species to identify molecular markers that are specific for these bacteria and which are also helpful in understanding their evolutionary relationships. We report here for the first time 13 molecular signatures consisting of conserved indels in widely distributed proteins that are distinctive characteristics of all sequenced Xanthomonadales species, but they are not found in any other bacteria. In view of their Xanthomonadales-specificity, the most parsimonious explanation to account for these CSIs is that the rare genetic changes responsible for them occurred only once in a common ancestor of the Xanthomonadales and were then passed on to various descendent species vertically as shown in Figure 9 [37], [54], [61]. Further, the absence of these CSIs in all other bacteria strongly indicates that the genes for these proteins have not been laterally transferred from Xanthomonadales to other bacterial groups or vice versa. Thus, these molecular signatures (or synapomorphies) provide novel means for the identification and circumscription of species from the order Xanthomonadales in clear molecular terms.
Figure 9. A summary diagram showing the species specificity of various CSIs identified in this work and the evolutionary stages where the genetic changes responsible for them were likely introduced.
We also report in this work detailed phylogenetic analyses of (sequenced) Xanthomonadales species based upon concatenated sequences for 28 widely distributed proteins. Earlier phylogenetic studies on Xanthomonadales are mainly based upon 16S rRNA or single genes such as Gyrase B and most of them cover only the genus Xanthomonas [4], [15], [59], [62], [63]. Among a number of novel relationships seen in this tree, these trees showed that Rhodanobacter sp. 2APBS1 formed the deepest branch within the Xanthomonadales and it was separated from all other species by a long branch. The branching of Pseudoxanthomonas and then other Xanthomonadaceae genera followed it. Importantly, our analyses have also identified 15 CSIs that are uniquely present in all other Xanthomonadales, except Rhodanobacter and in a few cases also by the Pseudoxanthomonas species. The genetic changes responsible for theses CSIs were likely introduced in a common ancestor of the other Xanthomonadales after the branching of Rhodanobacter and also in some cases Pseudoxanthomonas (Figure 9) and they provide independent evidence for the deep branching of these lineages with respect to other genera within this order.
Xanthomonadales species are indicated to have undergone extensive LGTs with other prokaryotic taxa particularly Alpha, Beta and some orders of Gamma- proteobacteria and in some cases with Archaea as well [16], [24]–[27]. In the present work, we have also identified several examples where a given CSI, in addition to being shared by all or most Xanthomonadales, was also present in some species from other groups of bacteria, most commonly from Alpha-, Beta- and Gamma- proteobacteria. Of these CSIs, five were present only in 1–3 species from other deep branching orders of Gammaproteobacteria and their possible significance is discussed below. Seven other CSIs were commonly shared by various Xanthomonadales and also several Betaproteobacteria and/or both Alpha- and Beta- proteobacteria. The shared presence of these CSIs between Xanthomonadales and these other proteobacteria could result from a number of possibilities including later transfer of the corresponding genes between these groups of bacteria or independent occurrence of similar genetic changes in these groups. However, phylogenetic trees based upon these protein sequences showed that Xanthomonadales species and the Alpha- and/or Beta- proteobacteria containing similar CSIs branched separately from each other, indicating that the presence of similar CSIs in these groups of bacteria was not due to LGTs. Therefore, genetic changes leading to similar CSIs in these groups likely occurred independently due to similar functional requirements for these CSIs. Although in our work we have not come across many examples of LGTs between Xanthomonadales and other groups of bacteria, our analyses is based only on proteins that contain conserved indels. Such genes/proteins represent only a small fraction of the total genes that are found in various genomes. Because most of these proteins are involved in essential functions, they are less prone to LGTs. In contrast, extensive work that Menck and coworkers have carried out on identification of cases of LGTs is primarily on species from the genus Xanthomonas [16], [24]–[27], which have thus far not studied in detail.
Xanthomonadales is one of the deepest branching orders within the Class Gammaproteobacteria. Some of the other orders that branch in its proximity include Chromatiales, Methylococcales, Cardiobacteriales, Legionelalles and Thiotrichales. However, the relationship of Xanthomonadales to these other orders is presently not understood. In the present work, we also identified six other CSIs (Table 2, last six entries), which in addition to various Xanthomonadales were also uniquely shared by 1 or 2 species from these orders of Gammaproteobacteria. The shared presence of these CSIs by Xanthomonadales and some of these other orders of Gammaproteobacteria suggests that either these orders are closely related or that similar genetic changes have occurred in them independently. However, further information from additional species from these orders will be necessary to establish whether the Xanthomonadales and some of these other orders of Gammaproteobacteria are specifically related and form a higher taxonomic clade within the Gammaproteobacteria.
The focus of the present study was on identifying molecular signatures that are specific for either the entire Xanthomonadales order or some of its deep branching lineages. Thus far, we have not carried out careful analyses of various signature sequences that are specific for specific genera viz. Xylella, Xanthomonas, Stenotrophomonas and Pseudoxanthomonas and such studies will be part of our future work. Nonetheless, based upon the identified molecular signatures it is now possible to identify and circumscribe species from the order Xanthomonadales from all other bacteria in clear molecular terms based upon large numbers of discrete molecular characteristics. Based upon our earlier work on CSIs for other groups/phyla of bacteria, most of these CSIs have degree of predictive ability [21], [64]–[66] and thus they are useful in identifying both known as well as unknown species belonging to these clades (viz. Xanthomonadales) in different environments. Xanthomonadales harbor many important plant pathogens that cause a variety of diseases in economically important crops and plants [3]–[9]. In addition, they also contain Stenotrophomonas, which are opportunistic human pathogens [12]–[14]. Thus, novel methods for sensitive and specific identification of species from this order in different settings are of much importance. Most of the Xanthomonadales-specific CSIs discovered in this work are present in highly conserved regions of the genes/proteins. Hence, based upon these gene sequences degenerate PCR primers (based upon either flanking conserved regions or the indel region and a flanking conserved region) could be readily designed to examine the presence or absence of gene sequences containing these CSIs in any given sample [64], [67]. Thus, molecular probes based upon these CSIs and/or their flanking regions should provide novel and specific means for the detection of new as well as existing Xanthomonadales species in different environments. The Xanthomonadales-specific CSIs, in addition to their usefulness for evolutionary and diagnostic studies, also provide novel and useful tools for genetic and biochemical investigations and possible means for identification of agents that specifically target these plant pathogenic bacteria.
Supporting Information
A maximum-likelihood tree based upon concatenated sequences for 28 conserved proteins. The tree shows the branching of Xanthomonadales group with Gammaproteobacteria. The tree was rooted using Alphaproteobacteria. The numbers on the nodes indicate statistical support for the nodes.
(PDF)
Partial sequence alignment of a conserved region in the amino acid/peptide transporter showing a 7 aa insert that is specific for all Xanthomonadales.
(PDF)
Partial sequence alignment of large-conductance mechanosensitive channel protein showing the presence of a 5 aa insert that is commonly shared by Xanthomonadales.
(PDF)
Partial sequence alignment of lysyl-tRNA synthetase showing a 3 aa insert that is uniquely shared by all members of Xanthomonadales.
(PDF)
Partial sequence alignment of lipoyl synthase showing a 2 aa insert that is commonly shared by Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region in the queuine tRNA-ribosyltransferase showing a 1 aa insert that is specific for Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region in the acyl-(acyl-carrier-protein)–UDP-N-acetylglucosamine O-acyltransferase showing a 1 aa insert that is commonly shared by Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region in the TolQ protein showing a 1 aa insert that is commonly shared by Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region in alpha-2-macroglobulin domain-containing protein showing a 13 aa deletion that is uniquely present in Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region of DNA topoisomerase IV subunit B showing a 1 aa deletion that is commonly specifically found in Xanthomonadales.
(PDF)
Partial sequence alignment of DNA polymerase I showing a 1 aa deletion that is uniquely shared by all members of Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region of aromatic amino acid aminotransferase showing a 1 aa deletion that is uniquely shared by Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region of DNA polymerase III subunit beta showing a 1 aa deletion that is present in Xanthomonadales. The CSI has also been found to be shared by Marinomonas sp. MWYL1 and Thioalkalivibrio sp. HL-EbGR7.
(PDF)
Partial sequence alignment of a conserved region of lipid-A-disaccharide synthase a 2 aa insert that is present in Xanthomonadales. The CSI has also been found to be shared by Cardiobacterium hominis, Allochromatium vinosum and Alteromonadales bacterium.
(PDF)
Partial sequence alignment of carbamoyl phosphate synthase large subunit a 1 aa insert that is present in Xanthomonadales. The CSI has also been found to be shared by Marinobacter sp. ELB17.
(PDF)
Partial sequence alignment of putative secreted protein showing a 1 aa insert that is present in Xanthomonadales. The CSI has also been found to be shared by Teredinibacter turnerae.
(PDF)
Partial sequence alignment of a conserved region of aminopeptidase P, showing a 1 aa deletion that is commonly shared by Xanthomonadales. The CSI has also been found to be shared by Thioalkalivibrio sp. HL-EbGR7 and Alkalilimnicola ehrlichii.
(PDF)
Partial sequence alignment of protoheme IX farnesyltransferase showing a 4 aa insert that is uniquely shared by subclade of Xanthomonadales after the divergence of Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of DNA polymerase III subunit alpha, showing a 1 aa insert that is uniquely present in Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region in the DEAD box helicase domain-containing protein showing a 1 aa insert that is specific for Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region in ribose-5-phosphate isomerase A, showing a 1 aa insert that is commonly shared by Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region in DNA polymerase I, showing a 1 aa insert that is uniquely shared by a subclade of Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of aspartyl-tRNA synthetase, showing a 4 aa deletion that is commonly shared by all Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region of 2-oxoglutarate-dehydrogenase E1 component, showing a 1 aa deletion that is unique to Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region of coproporphyrinogen III oxidase, showing a 1 aa deletion that is unique to Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region in 2-oxoglutarate-dehydrogenase E1 component, showing a 1 aa deletion that is uniquely shared by Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region of asparagine synthase b protein that is showing a 4–5 aa insert, unique to Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region of Asparagine synthase b protein, showing a 1–2 aa insert that is uniquely shared by Xanthomonadales except Rhodanobacter sp. 2APBS1. While genus Stenotrophomonas can be differentiated from other Xanthomonadales because of having 1 aa insert instead of 2 aa.
(PDF)
Partial sequence alignment of a conserved region DNA polymerase III subunit alpha showing a 4 aa insert that is commonly shared by Xanthomonadales except Rhodanobacter sp. 2APBS1. This CSI was previously identified by [28] as all Xanthomonadales specific signature.
(PDF)
Partial sequence alignment of a conserved region of RecA showing a 2 aa insert that is commonly shared by Xanthomonadales except Rhodanobacter sp. 2APBS1. This CSI was previously identified by [28] as all Xanthomonadales specific signature.
(PDF)
Partial sequence alignment of a conserved region of MutS showing a 5 aa insert that is commonly shared by Xanthomonadales except Pseudoxanthomonas suwonensis and Rhodanobacter sp. 2APBS1. This CSI was previously identified by [28] as all Xanthomonadales specific signature.
(PDF)
Partial sequence alignment of a conserved region of DNA polymerase III subunit alpha, showing a 2 aa deletion that is commonly shared by all Xanthomonadales except Pseudoxanthomonas suwonensis and Rhodanobacter sp. 2APBS1. These two have only 1 aa deletion at the same position.
(PDF)
Partial sequence alignment of tRNA (guanine-N(1)-)-methyltransferase, showing a 2 aa insert that is commonly present in all members of Xanthomonadales except Pseudoxanthomonas suwonensis and Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region in glucose-6-phosphate 1-dehydrogenase, showing a 4 aa deletion that is uniquely present in all Xanthomonadales except Pseudoxanthomonas spadix BD-a59 and Rhodanobacter sp. 2APBS1 which has 3 aa insert.
(PDF)
Partial sequence alignment of a conserved region of DNA ligase NAD dependent, showing a 57–65 aa insert that is commonly shared by all Xanthomonadales except Pseudoxanthomonas suwonensis and Rhodanobacter sp. 2APBS1. Both these species do not contain the insert of same length. This CSI was previously identified by [28] as all Xanthomonadales specific signature.
(PDF)
A Neighbor-joining tree based upon carbamoyl phosphate synthase large subunit sequence. The Tree is showing the distinct branching of Xanthomonadales from various β-Proteobacteria with and without insert.
(PDF)
Partial sequence alignment of a conserved region of Hypothetical protein XOO1065, showing a 1 aa deletion that is present in Xanthomonadales. The deletion has also been found to be shared by few species from β-Proteobacteria but not in all of them.
(PDF)
Partial sequence alignment of a conserved region of orotate phosphoribosyltransferase, showing a 1 aa deletion that is present in all Xanthomonadales. The deletion has also been found to be shared by species from β-Proteobacteria.
(PDF)
Partial sequence alignment of a conserved region of Putative ribonuclease HII, showing a 1 aa insert that is present in Xanthomonadales. The CSI has also been found to be shared by few species from β-Proteobacteria but is not present in all.
(PDF)
Partial sequence alignment of a conserved region of glycyl-tRNA synthetase subunit beta, showing a 2 aa insert that is present in Xanthomonadales. The insert has also been found to be shared by some β-Proteobacteria.
(PDF)
Partial sequence alignment of a conserved region of the septum-site determining protein MinD, showing a 1 aa deletion that is present in Xanthomonadales. This CSI is also present in some species from β-Proteobacteria.
(PDF)
A Neighbor-joining tree based upon sequences from hypothetical protein X001065. The Tree is showing the Xanthomonadales and various β-Proteobacteria that share the 1 aa deletion. Species representing some other Gammaproteobacteria are also shown in tree.
(PDF)
A Neighbor-joining tree for Proteobacterial species based upon orotate phosphoribosyltransferase sequences. The Xanthomonadales and different β-Proteobacteria that contain the 1 aa deletion in this protein do not branch together in this tree suggesting that the deletion in these two groups have likely occurred independently. The tree shows only representative species from other Gammaproteobacteria and Alphaproteobacteria and it was rooted using sequences from Epsilonproteobacteria.
(PDF)
A Neighbor-joining tree based upon sequences from putative ribonuclease HII. The Tree is showing the Xanthomonadales and various β-Proteobacteria with insert. The tree also shows representative species from other Gammaproteobacteria and Alphaproteobacteria.
(PDF)
A maximum-likelihood tree based upon sequences from glycyl-tRNA synthetase subunit beta. The Tree shows the branching of Xanthomonadales separately from the other insert containing Betaproteobacteria. The species distribution of this insert could be explained by either the independent occurrence of a similar genetic event in the Betaproteobacteria and the Xanthomonadales, or that this insert was introduced in a common ancestor of the Beta- and Gamma-proteobacteria, followed by its loss from other Gammaproteobacteria after the divergence of deep-branching Xanthomonadales.
(PDF)
A Neighbor-joining tree based upon sequences from septum site-determining protein MinD protein. The Tree is showing the branching of Xanthomonadales distinctly from the other insert containing Betaproteobacteria.
(PDF)
Funding Statement
NSERC (Natural Science and Engineering Research Council of Canada) 249924. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Saddler GS, Bradbury J.S (2005) “Order Xanthomonadales” Brenner DJ, Krieg NR, Staley JT editors Bergey’s Manual of Systematic Bacteriology, Vol. 2, 2nd edition, New York: Springer.
- 2.Brenner DJ, Krieg NR, Staley JT (2005) Bergey’s Manual of Systematic Bacteriology (2005) 2nd edition, Vol. 2, The Proteobacteria, New York: Springer.
- 3. Chatterjee S, Almeida RP, Lindow S (2008) Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Annu Rev Phytopathol 46: 243–271. [DOI] [PubMed] [Google Scholar]
- 4. Ryan RP, Vorholter FJ, Potnis N, Jones JB, Van Sluys MA, et al. (2011) Pathogenomics of Xanthomonas: understanding bacterium-plant interactions. Nat Rev Microbiol 9: 344–355. [DOI] [PubMed] [Google Scholar]
- 5. Lee BM, Park YJ, Park DS, Kang HW, Kim JG, et al. (2005) The genome sequence of Xanthomonas oryzae pathovar oryzae KACC10331, the bacterial blight pathogen of rice. Nucleic Acids Res 33: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salzberg SL, Sommer DD, Schatz MC, Phillippy AM, Rabinowicz PD, et al. (2008) Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99A. BMC Genomics 9: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Sluys MA, Monteiro-Vitorello CB, Camargo LE, Menck CF, da Silva AC, et al. (2002) Comparative genomic analysis of plant-associated bacteria. Annu Rev Phytopathol 40: 169–189. [DOI] [PubMed] [Google Scholar]
- 8. Bhattacharyya A, Stilwagen S, Ivanova N, D’Souza M, Bernal A, et al. (2002) Whole-genome comparative analysis of three phytopathogenic Xylella fastidiosa strains. Proc Natl Acad Sci U S A 99: 12403–12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Purcell AH, Hopkins DL (1996) Fastidious xylem-limited bacterial plant pathogens. Annu Rev Phytopathol 34: 131–151. [DOI] [PubMed] [Google Scholar]
- 10. Chen J, Xie G, Han S, Chertkov O, Sims D, et al. (2010) Whole genome sequences of two Xylella fastidiosa strains (M12 and M23) causing almond leaf scorch disease in California. J Bacteriol 192: 4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monteiro-Vitorello CB, de Oliveira MC, Zerillo MM, Varani AM, Civerolo E, et al. (2005) Xylella and Xanthomonas Mobil’omics. OMICS 9: 146–159. [DOI] [PubMed] [Google Scholar]
- 12. Crossman LC, Gould VC, Dow JM, Vernikos GS, Okazaki A, et al. (2008) The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol 9: R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Looney WJ, Narita M, Muhlemann K (2009) Stenotrophomonas maltophilia: an emerging opportunist human pathogen. Lancet Infect Dis 9: 312–323. [DOI] [PubMed] [Google Scholar]
- 14. Waters V, Yau Y, Prasad S, Lu A, Atenafu E, et al. (2011) Stenotrophomonas maltophilia in Cystic Fibrosis: Serologic Response and Effect on Lung Disease. Am J Respir Crit Care Med 183: 635–640. [DOI] [PubMed] [Google Scholar]
- 15. Yarza P, Ludwig W, Euzeby J, Amann R, Schleifer KH, et al. (2010) Update of the All-Species Living Tree Project based on 16S and 23S rRNA sequence analyses. Syst Appl Microbiol 33: 291–299. [DOI] [PubMed] [Google Scholar]
- 16. Martins-Pinheiro M, Galhardo RS, Lage C, Lima-Bessa KM, Aires KA, et al. (2004) Different patterns of evolution for duplicated DNA repair genes in bacteria of the Xanthomonadales group. BMC Evol Biol 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Comas I, Moya A, Azad RK, Lawrence JG, Gonzalez-Candelas F (2006) The evolutionary origin of Xanthomonadales genomes and the nature of the horizontal gene transfer process. Mol Biol Evol 23: 2049–2057. [DOI] [PubMed] [Google Scholar]
- 18. Dutilh BE, Huynen MA, Bruno WJ, Snel B (2004) The consistent phylogenetic signal in genome trees revealed by reducing the impact of noise. J Mol Evol 58: 527–539. [DOI] [PubMed] [Google Scholar]
- 19. Gupta RS, Sneath PHA (2007) Application of the character compatibility approach to generalized molecular sequence data: Branching order of the proteobacterial subdivisions. J Mol Evol 64: 90–100. [DOI] [PubMed] [Google Scholar]
- 20. Schneider A, Dessimoz C, Gonnet GH (2007) OMA Browser–exploring orthologous relations across 352 complete genomes. Bioinformatics 23: 2180–2182. [DOI] [PubMed] [Google Scholar]
- 21. Gao B, Mohan R, Gupta RS (2009) Phylogenomics and protein signatures elucidating the evolutionary relationships among the Gammaproteobacteria . Int J Syst Evol Microbiol 59: 234–247. [DOI] [PubMed] [Google Scholar]
- 22. Williams KP, Gillespie JJ, Sobral BW, Nordberg EK, Snyder EE, et al. (2010) Phylogeny of gammaproteobacteria. J Bacteriol 192: 2305–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gupta RS (2000) The phylogeny of Proteobacteria: relationships to other eubacterial phyla and eukaryotes. FEMS Microbiol Rev 24: 367–402. [DOI] [PubMed] [Google Scholar]
- 24. Lima WC, Van Sluys MA, Menck CF (2005) Non-gamma-proteobacteria gene islands contribute to the Xanthomonas genome. OMICS 9: 160–172. [DOI] [PubMed] [Google Scholar]
- 25. Lima WC, Paquola AC, Varani AM, Van Sluys MA, Menck CF (2008) Laterally transferred genomic islands in Xanthomonadales related to pathogenicity and primary metabolism. FEMS Microbiol Lett 281: 87–97. [DOI] [PubMed] [Google Scholar]
- 26. Lima WC, Menck CF (2008) Replacement of the arginine biosynthesis operon in Xanthomonadales by lateral gene transfer. J Mol Evol 66: 266–275. [DOI] [PubMed] [Google Scholar]
- 27. Lima WC, Varani AM, Menck CF (2009) NAD biosynthesis evolution in bacteria: lateral gene transfer of kynurenine pathway in Xanthomonadales and Flavobacteriales. Mol Biol Evol 26: 399–406. [DOI] [PubMed] [Google Scholar]
- 28. Cutino-Jimenez AM, Martins-Pinheiro M, Lima WC, Martin-Tornet A, Morales OG, et al. (2010) Evolutionary placement of Xanthomonadales based on conserved protein signature sequences. Mol Phylogenet Evol 54: 524–534. [DOI] [PubMed] [Google Scholar]
- 29. Thieme F, Koebnik R, Bekel T, Berger C, Boch J, et al. (2005) Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J Bacteriol 187: 7254–7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qian W, Jia Y, Ren SX, He YQ, Feng JX, et al. (2005) Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris. Genome Res 15: 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Potnis N, Krasileva K, Chow V, Almeida NF, Patil PB, et al. (2011) Comparative genomics reveals diversity among xanthomonads infecting tomato and pepper. BMC Genomics 12: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pieretti I, Royer M, Barbe V, Carrere S, Koebnik R, et al. (2009) The complete genome sequence of Xanthomonas albilineans provides new insights into the reductive genome evolution of the xylem-limited Xanthomonadaceae. BMC Genomics 10: 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bogdanove AJ, Koebnik R, Lu H, Furutani A, Angiuoli SV, et al. (2011) Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp. J Bacteriol 193: 5450–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Sluys MA, de Oliveira MC, Monteiro-Vitorello CB, Miyaki CY, Furlan LR, et al. (2003) Comparative analyses of the complete genome sequences of Pierce’s disease and citrus variegated chlorosis strains of Xylella fastidiosa. J Bacteriol 185: 1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simpson AJ, Reinach FC, Arruda P, Abreu FA, Acencio M, et al. (2000) The genome sequence of the plant pathogen Xylella fastidiosa. The Xylella fastidiosa Consortium of the Organization for Nucleotide Sequencing and Analysis. Nature 406: 151–159. [DOI] [PubMed] [Google Scholar]
- 36. Doddapaneni H, Yao J, Lin H, Walker MA, Civerolo EL (2006) Analysis of the genome-wide variations among multiple strains of the plant pathogenic bacterium Xylella fastidiosa. BMC Genomics 7: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gupta RS (1998) Protein phylogenies and signature sequences: A reappraisal of evolutionary relationships among archaebacteria, eubacteria, and eukaryotes. Microbiol Mol Biol Rev 62: 1435–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gupta RS, Griffiths E (2002) Critical issues in bacterial phylogeny. Theor Popul Biol 61: 423–434. [DOI] [PubMed] [Google Scholar]
- 39. Griffiths E, Gupta RS (2006) Molecular signatures in protein sequences that are characteristics of the Phylum Aquificales. Int J Syst Evol Microbiol 56: 99–107. [DOI] [PubMed] [Google Scholar]
- 40. Gupta RS (2009) Protein signatures (molecular synapomorphies) that are distinctive characteristics of the major cyanobacterial clades. Int J Syst Evol Microbiol 59: 2510–2526. [DOI] [PubMed] [Google Scholar]
- 41. Rokas A, Holland PW (2000) Rare genomic changes as a tool for phylogenetics. Trends Ecol Evol 15: 454–459. [DOI] [PubMed] [Google Scholar]
- 42. Baldauf SL, Palmer JD (1993) Animals and fungi are each other’s closest relatives: congruent evidence from multiple proteins. Proc Natl Acad Sci USA 90: 11558–11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rivera MC, Lake JA (1992) Evidence that eukaryotes and eocyte prokaryotes are immediate relatives. Science 257: 74–76. [DOI] [PubMed] [Google Scholar]
- 44. Gupta RS (2010) Molecular signatures for the main phyla of photosynthetic bacteria and their subgroups. Photosynth Res 104: 357–372. [DOI] [PubMed] [Google Scholar]
- 45. Gupta RS (2001) The branching order and phylogenetic placement of species from completed bacterial genomes, based on conserved indels found in various proteins. Int Microbiol 4: 187–202. [DOI] [PubMed] [Google Scholar]
- 46. Griffiths E, Gupta RS (2006) Lateral transfers of serine hydroxymethyl transferase (glyA) and UDP-N-acetylglucosamine enolpyruvyl transferase (murA) genes from free-living Actinobacteria to the parasitic chlamydiae. J Mol Evol 63: 283–296. [DOI] [PubMed] [Google Scholar]
- 47. Harris JK, Kelley ST, Spiegelman GB, Pace NR (2003) The genetic core of the universal ancestor. Genome Res 13: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ciccarelli FD, Doerks T, von Mering C, Creevey CJ, Snel B, et al. (2006) Toward automatic reconstruction of a highly resolved tree of life. Science 311: 1283–1287. [DOI] [PubMed] [Google Scholar]
- 49. Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with Clustal x. Trends Biochem Sci 23: 403–405. [DOI] [PubMed] [Google Scholar]
- 50. Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17: 540–552. [DOI] [PubMed] [Google Scholar]
- 51. Jones DT, Taylor WR, Thornton JM (1992) The rapid generation of mutation data matrices from protein sequences. Computer applications in the biosciences : CABIOS 8: 275–282. [DOI] [PubMed] [Google Scholar]
- 52. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 53. Whelan S, Goldman N (2001) A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol 18: 691–699. [DOI] [PubMed] [Google Scholar]
- 54. Naushad HS, Gupta RS (2012) Molecular signatures (conserved indels) in protein sequences that are specific for the order Pasteurellales and distinguish two of its main clades. Antonie van Leeuwenhoek 101: 105–124. [DOI] [PubMed] [Google Scholar]
- 55. Jalan N, Aritua V, Kumar D, Yu F, Jones JB, et al. (2011) Comparative genomic analysis of Xanthomonas axonopodis pv. citrumelo F1, which causes citrus bacterial spot disease, and related strains provides insights into virulence and host specificity. J Bacteriol 193: 6342–6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. da Silva AC, Ferro JA, Reinach FC, Farah CS, Furlan LR, et al. (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417: 459–463. [DOI] [PubMed] [Google Scholar]
- 57. Schreiber HL, Koirala M, Lara A, Ojeda M, Dowd SE, et al. (2010) Unraveling the First Xylella fastidiosa Subsp. Fastidiosa Genome from Texas. Southwestern Entomologist 35: 479–483. [Google Scholar]
- 58. Lee SH, Jin HM, Lee HJ, Kim JM, Jeon CO (2012) Complete Genome Sequence of the BTEX-Degrading Bacterium Pseudoxanthomonas spadix BD-a59. J Bacteriol 194: 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Parkinson N, Cowie C, Heeney J, Stead D (2009) Phylogenetic structure of Xanthomonas determined by comparison of gyrB sequences. Int J Syst Evol Microbiol 59: 264–274. [DOI] [PubMed] [Google Scholar]
- 60. Handy J, Doolittle RF (1999) An attempt to pinpoint the phylogenetic introduction of glutaminyl-tRNA synthetase among bacteria. Journal of Molecular Evolution 49: 709–715. [DOI] [PubMed] [Google Scholar]
- 61. Gupta RS, Mathews DW (2010) Signature proteins for the major clades of Cyanobacteria . BMC Evol Biol 10: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Parkinson N, Aritua V, Heeney J, Cowie C, Bew J, et al. (2007) Phylogenetic analysis of Xanthomonas species by comparison of partial gyrase B gene sequences. Int J Syst Evol Microbiol 57: 2881–2887. [DOI] [PubMed] [Google Scholar]
- 63. Young JM, Park DC, Shearman HM, Fargier E (2008) A multilocus sequence analysis of the genus Xanthomonas. Syst Appl Microbiol 31: 366–377. [DOI] [PubMed] [Google Scholar]
- 64. Gao B, Gupta RS (2005) Conserved indels in protein sequences that are characteristic of the phylum Actinobacteria . Int J Syst Evol Microbiol 55: 2401–2412. [DOI] [PubMed] [Google Scholar]
- 65. Gupta RS (2011) Origin of diderm (Gram-negative) bacteria: antibiotic selection pressure rather than endosymbiosis likely led to the evolution of bacterial cells with two membranes. Antonie van Leeuwenhoek 100: 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gao B, Gupta RS (2012) Microbial systematics in the post-genomics era. Antonie van Leeuwenhoek 101: 45–54. [DOI] [PubMed] [Google Scholar]
- 67. Griffiths E, Gupta RS (2002) Protein signatures distinctive of chlamydial species: Horizontal transfer of cell wall biosynthesis genes glmU from Archaebacteria to Chlamydiae, and murA between Chlamydiae and Streptomyces . Microbiology 148: 2541–2549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A maximum-likelihood tree based upon concatenated sequences for 28 conserved proteins. The tree shows the branching of Xanthomonadales group with Gammaproteobacteria. The tree was rooted using Alphaproteobacteria. The numbers on the nodes indicate statistical support for the nodes.
(PDF)
Partial sequence alignment of a conserved region in the amino acid/peptide transporter showing a 7 aa insert that is specific for all Xanthomonadales.
(PDF)
Partial sequence alignment of large-conductance mechanosensitive channel protein showing the presence of a 5 aa insert that is commonly shared by Xanthomonadales.
(PDF)
Partial sequence alignment of lysyl-tRNA synthetase showing a 3 aa insert that is uniquely shared by all members of Xanthomonadales.
(PDF)
Partial sequence alignment of lipoyl synthase showing a 2 aa insert that is commonly shared by Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region in the queuine tRNA-ribosyltransferase showing a 1 aa insert that is specific for Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region in the acyl-(acyl-carrier-protein)–UDP-N-acetylglucosamine O-acyltransferase showing a 1 aa insert that is commonly shared by Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region in the TolQ protein showing a 1 aa insert that is commonly shared by Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region in alpha-2-macroglobulin domain-containing protein showing a 13 aa deletion that is uniquely present in Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region of DNA topoisomerase IV subunit B showing a 1 aa deletion that is commonly specifically found in Xanthomonadales.
(PDF)
Partial sequence alignment of DNA polymerase I showing a 1 aa deletion that is uniquely shared by all members of Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region of aromatic amino acid aminotransferase showing a 1 aa deletion that is uniquely shared by Xanthomonadales.
(PDF)
Partial sequence alignment of a conserved region of DNA polymerase III subunit beta showing a 1 aa deletion that is present in Xanthomonadales. The CSI has also been found to be shared by Marinomonas sp. MWYL1 and Thioalkalivibrio sp. HL-EbGR7.
(PDF)
Partial sequence alignment of a conserved region of lipid-A-disaccharide synthase a 2 aa insert that is present in Xanthomonadales. The CSI has also been found to be shared by Cardiobacterium hominis, Allochromatium vinosum and Alteromonadales bacterium.
(PDF)
Partial sequence alignment of carbamoyl phosphate synthase large subunit a 1 aa insert that is present in Xanthomonadales. The CSI has also been found to be shared by Marinobacter sp. ELB17.
(PDF)
Partial sequence alignment of putative secreted protein showing a 1 aa insert that is present in Xanthomonadales. The CSI has also been found to be shared by Teredinibacter turnerae.
(PDF)
Partial sequence alignment of a conserved region of aminopeptidase P, showing a 1 aa deletion that is commonly shared by Xanthomonadales. The CSI has also been found to be shared by Thioalkalivibrio sp. HL-EbGR7 and Alkalilimnicola ehrlichii.
(PDF)
Partial sequence alignment of protoheme IX farnesyltransferase showing a 4 aa insert that is uniquely shared by subclade of Xanthomonadales after the divergence of Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of DNA polymerase III subunit alpha, showing a 1 aa insert that is uniquely present in Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region in the DEAD box helicase domain-containing protein showing a 1 aa insert that is specific for Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region in ribose-5-phosphate isomerase A, showing a 1 aa insert that is commonly shared by Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region in DNA polymerase I, showing a 1 aa insert that is uniquely shared by a subclade of Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of aspartyl-tRNA synthetase, showing a 4 aa deletion that is commonly shared by all Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region of 2-oxoglutarate-dehydrogenase E1 component, showing a 1 aa deletion that is unique to Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region of coproporphyrinogen III oxidase, showing a 1 aa deletion that is unique to Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region in 2-oxoglutarate-dehydrogenase E1 component, showing a 1 aa deletion that is uniquely shared by Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region of asparagine synthase b protein that is showing a 4–5 aa insert, unique to Xanthomonadales except Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region of Asparagine synthase b protein, showing a 1–2 aa insert that is uniquely shared by Xanthomonadales except Rhodanobacter sp. 2APBS1. While genus Stenotrophomonas can be differentiated from other Xanthomonadales because of having 1 aa insert instead of 2 aa.
(PDF)
Partial sequence alignment of a conserved region DNA polymerase III subunit alpha showing a 4 aa insert that is commonly shared by Xanthomonadales except Rhodanobacter sp. 2APBS1. This CSI was previously identified by [28] as all Xanthomonadales specific signature.
(PDF)
Partial sequence alignment of a conserved region of RecA showing a 2 aa insert that is commonly shared by Xanthomonadales except Rhodanobacter sp. 2APBS1. This CSI was previously identified by [28] as all Xanthomonadales specific signature.
(PDF)
Partial sequence alignment of a conserved region of MutS showing a 5 aa insert that is commonly shared by Xanthomonadales except Pseudoxanthomonas suwonensis and Rhodanobacter sp. 2APBS1. This CSI was previously identified by [28] as all Xanthomonadales specific signature.
(PDF)
Partial sequence alignment of a conserved region of DNA polymerase III subunit alpha, showing a 2 aa deletion that is commonly shared by all Xanthomonadales except Pseudoxanthomonas suwonensis and Rhodanobacter sp. 2APBS1. These two have only 1 aa deletion at the same position.
(PDF)
Partial sequence alignment of tRNA (guanine-N(1)-)-methyltransferase, showing a 2 aa insert that is commonly present in all members of Xanthomonadales except Pseudoxanthomonas suwonensis and Rhodanobacter sp. 2APBS1.
(PDF)
Partial sequence alignment of a conserved region in glucose-6-phosphate 1-dehydrogenase, showing a 4 aa deletion that is uniquely present in all Xanthomonadales except Pseudoxanthomonas spadix BD-a59 and Rhodanobacter sp. 2APBS1 which has 3 aa insert.
(PDF)
Partial sequence alignment of a conserved region of DNA ligase NAD dependent, showing a 57–65 aa insert that is commonly shared by all Xanthomonadales except Pseudoxanthomonas suwonensis and Rhodanobacter sp. 2APBS1. Both these species do not contain the insert of same length. This CSI was previously identified by [28] as all Xanthomonadales specific signature.
(PDF)
A Neighbor-joining tree based upon carbamoyl phosphate synthase large subunit sequence. The Tree is showing the distinct branching of Xanthomonadales from various β-Proteobacteria with and without insert.
(PDF)
Partial sequence alignment of a conserved region of Hypothetical protein XOO1065, showing a 1 aa deletion that is present in Xanthomonadales. The deletion has also been found to be shared by few species from β-Proteobacteria but not in all of them.
(PDF)
Partial sequence alignment of a conserved region of orotate phosphoribosyltransferase, showing a 1 aa deletion that is present in all Xanthomonadales. The deletion has also been found to be shared by species from β-Proteobacteria.
(PDF)
Partial sequence alignment of a conserved region of Putative ribonuclease HII, showing a 1 aa insert that is present in Xanthomonadales. The CSI has also been found to be shared by few species from β-Proteobacteria but is not present in all.
(PDF)
Partial sequence alignment of a conserved region of glycyl-tRNA synthetase subunit beta, showing a 2 aa insert that is present in Xanthomonadales. The insert has also been found to be shared by some β-Proteobacteria.
(PDF)
Partial sequence alignment of a conserved region of the septum-site determining protein MinD, showing a 1 aa deletion that is present in Xanthomonadales. This CSI is also present in some species from β-Proteobacteria.
(PDF)
A Neighbor-joining tree based upon sequences from hypothetical protein X001065. The Tree is showing the Xanthomonadales and various β-Proteobacteria that share the 1 aa deletion. Species representing some other Gammaproteobacteria are also shown in tree.
(PDF)
A Neighbor-joining tree for Proteobacterial species based upon orotate phosphoribosyltransferase sequences. The Xanthomonadales and different β-Proteobacteria that contain the 1 aa deletion in this protein do not branch together in this tree suggesting that the deletion in these two groups have likely occurred independently. The tree shows only representative species from other Gammaproteobacteria and Alphaproteobacteria and it was rooted using sequences from Epsilonproteobacteria.
(PDF)
A Neighbor-joining tree based upon sequences from putative ribonuclease HII. The Tree is showing the Xanthomonadales and various β-Proteobacteria with insert. The tree also shows representative species from other Gammaproteobacteria and Alphaproteobacteria.
(PDF)
A maximum-likelihood tree based upon sequences from glycyl-tRNA synthetase subunit beta. The Tree shows the branching of Xanthomonadales separately from the other insert containing Betaproteobacteria. The species distribution of this insert could be explained by either the independent occurrence of a similar genetic event in the Betaproteobacteria and the Xanthomonadales, or that this insert was introduced in a common ancestor of the Beta- and Gamma-proteobacteria, followed by its loss from other Gammaproteobacteria after the divergence of deep-branching Xanthomonadales.
(PDF)
A Neighbor-joining tree based upon sequences from septum site-determining protein MinD protein. The Tree is showing the branching of Xanthomonadales distinctly from the other insert containing Betaproteobacteria.
(PDF)