Abstract
WRKY proteins comprise one of the largest transcription factor families in plants and form key regulators of many plant processes. This study presents the characterization of 58 WRKY genes from the castor bean (Ricinus communis L., Euphorbiaceae) genome. Compared with the automatic genome annotation, one more WRKY-encoding locus was identified and 20 out of the 57 predicted gene models were manually corrected. All RcWRKY genes were shown to contain at least one intron in their coding sequences. According to the structural features of the present WRKY domains, the identified RcWRKY genes were assigned to three previously defined groups (I–III). Although castor bean underwent no recent whole-genome duplication event like physic nut (Jatropha curcas L., Euphorbiaceae), comparative genomics analysis indicated that one gene loss, one intron loss and one recent proximal duplication occurred in the RcWRKY gene family. The expression of all 58 RcWRKY genes was supported by ESTs and/or RNA sequencing reads derived from roots, leaves, flowers, seeds and endosperms. Further global expression profiles with RNA sequencing data revealed diverse expression patterns among various tissues. Results obtained from this study not only provide valuable information for future functional analysis and utilization of the castor bean WRKY genes, but also provide a useful reference to investigate the gene family expansion and evolution in Euphorbiaceus plants.
Introduction
WRKY transcription factors, defined by the presence of the conserved WRKY domain of approximate 60 amino acids, play an essential regulatory role in plant growth, development, metabolism, and biotic and abiotic stress responses [1–3]. Since the first WRKY-encoding gene was isolated from sweet potato (Ipomoea batatas) [4], its homologs have been found in a wide range of plants and several non-plant species including Giardia lamblia, Dictyostelium discoideum, diplomonads, social amoebae, fungi incertae sedis and amoebozoa [5,6]. Compared with low and non-plants, the WRKY genes in high plants were shown to be highly expanded. For example, there are 57 members in cucumber (Cucumis sativus), 58 in physic nut (Jatropha curcas), 59 in grapevine (Vitis vinifera), 72 in Arabidopsis thaliana, 103 in white pear (Pyrus bretschneideri), 105 in poplar (Populus trichocarpa), 105 in foxtail millet (Setaria italica) and more than 100 in rice (Oryza sativa) [7–14]. WRKY proteins contain one or two WRKY domains, comprising the highly conserved WRKYGQK heptapeptide at the N-termini and a novel zinc finger motif (Cx4–7Cx22–23HxH/C) at the C-termini [10]. Both of these two motifs are vital for the high binding affinity of the WRKY proteins to the consensus cis-acting element termed the W box (TTGACT/C) [15,16]. According to the number of WRKY domains and the features of their zinc finger motifs, WRKY proteins can be categorized into three main groups. The group I members have two WRKY domains and feature the zinc finger motif of C2H2. Both groups II and III members contain a single WRKY domain, and the group III members possess the C2HC zinc finger motif which is different from C2H2 as observed in groups I and II members. Base on the evolutionary relationship and certain amino acid motifs present outside the WRKY domain, the group II can be further divided into 5 subgroups (a–e) [10]. In contrast to the presence of a conserved PR intron located after the codon encoding arginine (N terminal to the zinc finger motif) of subgroups c-e as seen in the group III and the C-terminal WRKY domain of the group I, members of subgroups a and b harbor a VQR intron in the zinc finger motif instead [6,10,17].
Castor bean (Ricinus communis L.), a tropical perennial shrub that belongs to the Euphorbiaceae family, is one of the most important non-food oilseed crops cultivated for industrial, medicinal and cosmetic purposes. Although native to Africa, the economic importance of castor bean oil and its well-adaptation to unfavorable conditions has prompted its wide-domestication to many tropical, subtropical and warm temperate regions around the world [18,19]. Given the crucial role of WRKY transcription factors in plant adaptation, two independent groups performed the homology search against the recently available castor bean draft genome [20] for the RcWRKY genes [17,21]. The study performed by Li et al. [21] focused on the expression analysis of the 47 identified RcWRKY genes in roots, stems, leaves, male flowers, female flowers and fruits at different developmental stages (i.e. 7, 15, 30 and 45 days post-anthesis) by using quantitative real-time PCR (qRT-PCR). Another study carried out by Zou [17] described the identification of nine more family members (i.e. 56 RcWRKYs) based on the automatic annotation of the castor bean genome, mainly focusing on the analysis of the evolutionary relationships between RcWRKY members by using the conserved WRKY domains. However, when compared with physic nut, another Euphorbiaceae plant species without the occurrence of any recent whole-genome duplication as castor bean [20,22], the family number of castor bean [17] seems to be relatively small and several physic nut WRKY genes [7] have no counterparts in castor bean. These results suggest that the RcWRKY genes have not been fully identified or the loss of specific genes has occurred in the castor bean genome. Thereby, rechecking the RcWRKY gene family is still needed.
Along with the 4.6 × draft genome of castor bean, as of Apr 2015, 88212 nucleotides and 62629 expressed sequence tags (ESTs) have been deposited in NCBI GenBank. In addition, RNA sequencing data from several tissues such as root, leaf, flower, seed and endosperm is also available in NCBI SRA, which includes 1,138,884 Roche 454 reads and 386,847,526 Illumina reads [23–26]. These datasets provide a good chance to analyze the castor bean WRKY gene family from a global view. In the present study, we take advantage of the genome sequences and available transcriptome data to identify the complete set of the RcWRKY genes and conduct the expert revision of their gene structures via mapping the ESTs and RNA sequencing reads against the scaffolds. Further, the sequence characteristics, evolutionary relationships and transcriptional profiling of the identified RcWRKY genes were also investigated.
Methods
Datasets and sequence retrieval
Sequences of 72 Arabidopsis and 58 physic nut WRKY proteins described before [7,10] were obtained from TAIR (release 10, http://www.arabidopsis.org/) and NCBI (http://www.ncbi.nlm.nih.gov/), respectively (the accession number are available in S1 Table). The genome sequences and annotation information of castor bean [20] were downloaded from phytozome v10.2 (http://phytozome.jgi.doe.gov/pz/portal.html), whereas the nucleotides, Sanger ESTs and raw RNA sequencing reads were downloaded from NCBI.
Identification and manual curation of the castor bean WRKY genes
To obtain the complete set of castor bean WRKY genes, the tBlastn search [27] was performed using a representative WRKY domain from each WRKY subgroups (I, IIa, IIb, IIc, IId, IIe and III) and the e-value was set to 10. Positive genomic sequences were also analyzed using the HMMER program [28] and Hidden Markov Model (HMM) trained with RcWRKYs. The presence of WRKY domains in candidate RcWRKY proteins was confirmed using the SMART program (http://smart.embl-heidelberg.de/) [29]. The predicted gene models were further checked with ESTs and raw RNA sequencing reads. Gene structures were displayed using GSDS [30]. Homology search for nucleotides or ESTs was performed using Blastn [27] and sequences with a similarity of more than 98% were taken into account, whereas RNA sequencing clean reads (see below) were mapped using Bowtie 2 [31] with default parameters and mapped read number of more than one was counted as expressed. The alternative splicing isoforms were identified using Cufflinks (v2.2.1) [32]. In addition, the ortholog of each RcWRKY in Arabidopsis and physic nut was identified using Blastp [27] (e-value, 1e−20) against AtWRKYs and JcWRKYs, and the reciprocal Blastp was performed to confirm true orthologs. Tandem or proximal duplications were considered when two duplicated genes were consecutive in the genome or separated by 20 or fewer gene loci, respectively.
Sequence alignments, phylogenetic analysis and classification of RcWRKY genes
Multiple alignments were performed using MUSCLE [33]. The alignment of all RcWRKY domains were displayed using Boxshade (http://www.ch.embnet.org/software/BOX_form.html), whereas the alignment including Dictyostelium discoideum WRKY1 [5] (UniProtKB accession number Q554C5; the N and C-terminal WRKY domain was denoted as DdWRKY1N or DdWRKY1C, respectively; the same as for other group I members), RcWRKYs, AtWRKYs and JcWRKYs were used for phylogenetic tree construction. By using DdWRKY1C as an outgroup, the tree was constructed using MEGA 6.0 [34] with the maximum likelihood method and with the bootstrap test replicated 1000 times. Classification of RcWRKYs into groups and subgroups was done based on the structural features and evolutionary relationships of the WRKY domains.
Protein properties and conserved motif analysis
Protein properties of RcWRKYs, e.g., the molecular weight (MW), isoelectric point (pI), and grand average of hydropathicity (GRAVY) were calculated using ProtParam (http://web.expasy.org/protparam/). Analysis for conserved motifs in RcWRKY proteins was carried out using MEME (http://meme.sdsc.edu/meme/cgi-bin/meme.cgi) [35]. The optimized parameters were: any number of repetitions; maximum number of motifs, 15; and the optimum width of each motif, between 6 and 50 residues. Subsequently, the MAST program was used to search detected motifs in protein databases. The online software 2ZIP (http://2zip.molgen.mpg.de/index.html) was used to predict the conserved Leu zipper motif, whereas HARF, LxxLL (x, any amino acid) and LxLxLx motifs were identified manually.
Gene expression analyses
To analyze the global expression profiles of RcWRKY genes among different tissues or certain tissue of developmental stages, RNA sequencing data of leaf (NCBI SRA accession number ERX021378), flower (ERX021379), endosperm (ERX021375 and ERX021376) and seed (ERX021377) described before [24] were examined. The clean reads were obtained by removing adaptor sequences, adaptor-only reads, reads with “N” rate larger than 10% (“N” representing ambiguous bases) and low quality reads containing more than 50% bases with Q-value≤5. Then, the clean reads were mapped to the 58 identified RcWRKY genes (coding sequence, CDS) and released transcripts using Bowtie 2 [31], and the RPKM (reads per kilo bases per million reads) method [36] was used for the expression annotation. Unless specific statements, the tools used in this study were performed with default parameters.
Results and Discussion
Characterization of 58 WRKY-encoding sequences in castor bean
The homology search resulted in 58 loci putatively encoding WRKY genes from 41 scaffolds of the castor bean genome. Among them, 57 loci were predicted by the genome annotation [20] and further annotated by the PlantTFDB which used the released gene models for the annotation of RcWRKY genes [37], whereas one more loci encoding 117 residues was identified from the scaffold28842 (Table 1) and its ortholog was also found in physic nut [7]. Since the gene models of RcWRKY genes were the result of an automatic annotation due to the lack of transcriptome data at that time, an expert revision of their gene structures was conducted via mapping the ESTs and reads against the scaffolds. Interestingly enough, the results showed that 20 out of the 57 predicted gene models seem not to be properly annotated (Table 1). The locus 29929.t000090 was predicted to encode 609 residues which is relatively shorter than its ortholog in physic nut (JcWRKY10, 740 residues) [7], however, hundreds of RNA sequencing reads indicated that the “TTNNNTTGAC” sequence was misassembled into its first exon. Thereby, this locus is promised to harbor four introns putatively encoding 711 residues (see S1 File). The locus 29820.t000050 was predicted to encode 558 residues, however, read mapping indicated that partial sequences of its second and third exons were annotated as the second intron, thus this locus is promised to encode 598 residues (see S2 File) which is similar to that of its physic nut ortholog (JcWRKY08, 576 residues) [7]. As for the locus 29635.t000028, though both the predicted and identified CDSs encode 510 residues, read mapping indicated that the “GCAA” sequence of the second intron was annotated as the second exon and the “GCAG” sequence of the third exon was annotated as the second intron (see S3 File). The locus 30174.t000563 was predicted to encode 468 residues, however, read mapping and ORF (open reading frame) analysis suggested that it represents only the 3’ sequence of the gene which is promised to encode 524 residues (see S4 File). The locus 29687.t000003 was predicted to contain five introns encoding 503 residues, however, sequence analysis indicated that the N-terminal WRKY domain of the deduced protein is incomplete. EST and read mapping suggested that this locus is promised to harbor four introns and putatively encode 511 residues (see S5 File). The locus 29848.t000095 was predicted to have two introns encoding 372 residues, however, read mapping indicated that it represents only the 3’ sequence of this gene which is promised to harbor four introns putatively encoding 451 residues. In addition, its third exon was also misannotated as an intron (see S6 File). The locus 30174.t000066 was predicted to encode 192 residues, however, read mapping indicated that this locus is promised to encode 196 residues (see S7 File). The locus 28040.t000001 was predicted to contain a single intron encoding 103 residues, however, read mapping and ORF analysis suggested that it represents only the 3’ sequence of this gene which is promised to have two introns putatively encoding 217 residues (see S8 File). The locus 29709.t000007 was predicted to encode 185 residues, however, it didn’t contain the complete WRKY domain. Instead, read mapping indicated that this locus is promised to encode 205 residues (see S9 File). The locus 29889.t000087 was predicted to encode 351 residues, however, read mapping and ORF analysis suggested that it represents only the 3’ sequence of the gene which is promised to encode 360 residues (see S10 File). The locus 30174.t000532 was predicted to encode 313 residues, however, read mapping indicated that this locus is promised to encode 308 residues (see S11 File). The locus 43951.t000001 was predicted to encode 195 residues, however, EST and read mapping indicated that another locus 30131.t000001 from scaffold43951 (1019 bp) also belongs to this gene, and the gene is promised to harbor three introns putatively encoding 318 residues (see S12 File). The locus 29848.t000101 was predicted to encode 211 residues, however, EST and read mapping indicated that this locus is promised to encode 330 residues (see S13 File). The locus 29848.t000100 was predicted to contain three introns encoding 139 residues, however, sequence analysis revealed that its WRKY domain is incomplete. Further read mapping indicated that this locus is promised to harbor three introns putatively encoding 242 residues. The first exon and the first intron of this gene were not annotated previously, whereas partial sequences of its fourth exon were not annotated or misannotated as the third intron (see S14 File). The locus 29736.t000019 was predicted to contain three introns encoding 562 residues, however, read mapping indicated that this locus is promised to harbor four introns putatively encoding 634 residues (see S15 File). The locus 29598.t000004 was predicted to contain three introns encoding 263 residues, however, read mapping indicated that this locus is promised to harbor two introns putatively encoding 353 residues and partial sequence of its first exon was misannotated as an intron (see S16 File). The locus 29644.t000015 was predicted to contain one intron encoding 105 residues, however, EST and read mapping indicated that another locus 29644.t000016 on the same scaffold also belongs to this gene, and the misannotation was resulted from the “TCTTGCTCCAGAAGAG” sequence that was misassembled into its first exon. Thereby, this locus is promised to harbor two introns putatively encoding 356 residues (see S17 File). The locus 28455.t000009 was predicted to contain four introns encoding 367 residues, however, read mapping indicated that this locus is promised to harbor two introns putatively encoding 317 residues (see S18 File). The locus 27996.t000002 was predicted to contain four introns encoding 466 residues, however, read mapping indicated that this locus is promised to harbor two introns putatively encoding 480 residues, and partial sequences of its first exon and intron were misannotated as an intron or an exon, respectively (see S19 File). The locus 28690.t000001 was predicted to contain three introns encoding 287 residues, however, read mapping indicated that this locus is promised to harbor two introns putatively encoding 339 residues (see S20 File).
Table 1. List of the 58 RcWRKY genes identified in this study.
| Gene name | Scaffold ID | Predicted position | Locus ID | Transcript ID | Identified position | EST hits | Expressed | ASa | ASb | (Sub)group and comments | Deduced polypeptide | At_ortholog | Jc_ortholog | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Length (aa) | MW (kDa) | pI | GRAVY | |||||||||||||
| RcWRKY01 | scaffold29949 | 50158–52013 | 29949.t000007 | 29949.m000123 | 49111–52359 | - | Yes | - | Yes | I | 484 | 52.89 | 7.04 | -0.921 | AtWRKY01 | JcWRKY01 |
| RcWRKY02 | scaffold27613 | 207515–213650 | 27613.t000032 | 27613.m000639 | 207484–214016 | 2 | Yes | - | Yes | I | 562 | 60.78 | 6.84 | -0.789 | AtWRKY20 | JcWRKY09 |
| RcWRKY03 | scaffold29929 | 510214–512861 | 29929.t000090 | 29929.m004587 | 509685–512932 | - | Yes | - | - | I, misassembled | 711 | 77.45 | 5.66 | -0.675 | AtWRKY20 | JcWRKY10 |
| RcWRKY04 | scaffold28966 | 27612–23998 | 28966.t000003 | 28966.m000524 | 29398–23813 | - | Yes | - | Yes | I | 733 | 79.82 | 6.13 | -0.761 | AtWRKY02,34 | JcWRKY11 |
| RcWRKY05 | scaffold29717 | 13600–10576 | 29717.t000002 | 29717.m000222 | 13200–10572 | 43 | Yes | Yes | Yes | I | 575 | 63.47 | 6.71 | -1.043 | AtWRKY33,25,26 | JcWRKY07 |
| RcWRKY06 | scaffold29820 | 294372–291661 | 29820.t000050 | 29820.m001029 | 294438–289616 | - | Yes | - | Yes | I, misannotated | 598 | 65.52 | 7.59 | -0.770 | AtWRKY33,25,26 | JcWRKY08 |
| RcWRKY07 | scaffold29635 | 203409–198815 | 29635.t000028 | 29635.m000468 | 203640–198543 | 1 | Yes | - | Yes | I, misannotated | 510 | 55.69 | 7.33 | -0.812 | AtWRKY04,03 | JcWRKY06 |
| RcWRKY08 | scaffold30174 | 3380314–3384496 | 30174.t000563 | 30174.m009166 | 3380374–3384508 | 9 | Yes | - | - | I, misannotated | 524 | 57.12 | 7.75 | -0.837 | AtWRKY04,03 | JcWRKY05 |
| RcWRKY09 | scaffold29805 | 189773–191900 | 29805.t000035 | 29805.m001504 | 187614–192328 | - | Yes | - | Yes | I | 474 | 52.00 | 8.59 | -0.946 | AtWRKY44 | JcWRKY04 |
| RcWRKY10 | scaffold29687 | 15684–21009 | 29687.t000003 | 29687.m000562 | 15455–21624 | 1 | Yes | - | Yes | I, misannotated | 511 | 56.01 | 5.67 | -0.811 | AtWRKY32 | JcWRKY02 |
| RcWRKY11 | scaffold29848 | 493883–492201 | 29848.t000095 | 29848.m004539 | 494292–491256 | - | Yes | - | Yes | I, misannotated | 451 | 45.84 | 8.65 | -0.754 | AtWRKY32 | JcWRKY03 |
| RcWRKY12 | scaffold29771 | 4111–8719 | 29771.t000001 | 29771.m000072 | 4111–8868 | - | Yes | - | - | IIc | 215 | 24.33 | 6.83 | -0.966 | AtWRKY51,50,59,68 | JcWRKY12 |
| RcWRKY13 | scaffold29739 | 137722–136858 | 29739.t000022 | 29739.m003586 | 137722–136462 | - | Yes | - | Yes | IIc | 159 | 18.03 | 5.98 | -0.959 | AtWRKY50,51,59,68 | JcWRKY14 |
| RcWRKY14 | scaffold28644 | 112321–114401 | 28644.t000022 | 28644.m000915 | 112007–114711 | - | Yes | - | - | IIc | 168 | 19.40 | 5.49 | -1.001 | AtWRKY51,50,59,68 | JcWRKY13 |
| RcWRKY15 | scaffold29929 | 718638–720485 | 29929.t000127 | 29929.m004624 | 718359–720760 | - | Yes | - | - | IIc | 203 | 22.79 | 9.15 | -0.817 | AtWRKY75,45 | JcWRKY17 |
| RcWRKY16 | scaffold30190 | 612076–613585 | 30190.t000144 | 30190.m010908 | 611849–613809 | - | Yes | - | - | IIc | 194 | 22.28 | 9.30 | -0.844 | AtWRKY75,45 | JcWRKY18 |
| RcWRKY17 | scaffold30147 | 2203761–2204393 | 30147.t000745 | 30147.m014474 | 2203665–2204574 | - | Yes | - | - | IIc | 164 | 18.96 | 9.49 | -1.075 | AtWRKY75,45 | JcWRKY19 |
| RcWRKY18 | scaffold30174 | 2076040–2077754 | 30174.t000066 | 30174.m008669 | 2075781–2078009 | - | Yes | - | Yes | IIc, misannotated | 196 | 22.42 | 8.93 | -0.622 | AtWRKY43,24,56 | JcWRKY21 |
| RcWRKY19 | scaffold30190 | 3026476–3027219 | 30190.t000514 | 30190.m011278 | 3026363–3027328 | - | Yes | - | - | IIc | 185 | 21.06 | 9.01 | -0.661 | AtWRKY56,24,43 | JcWRKY20 |
| RcWRKY20 | scaffold28040 | 31334–28206 | 28040.t000001 | 28040.m000035 | 32051–27986 | - | Yes | - | - | IIc, misannotated | 217 | 24.87 | 9.34 | -0.724 | AtWRKY13 | JcWRKY15 |
| RcWRKY21 | scaffold29709 | 41452–43070 | 29709.t000007 | 29709.m001171 | 41208–44408 | - | Yes | - | Yes | IIc, misannotated | 205 | 23.60 | 7.07 | -1.020 | AtWRKY12 | JcWRKY16 |
| RcWRKY22 | scaffold29889 | 412384–414133 | 29889.t000087 | 29889.m003321 | 412309–414133 | 1 | Yes | - | - | IIc, misannotated | 360 | 39.98 | 6.32 | -0.793 | AtWRKY48 | JcWRKY23 |
| RcWRKY23 | scaffold29693 | 677606–676301 | 29693.t000098 | 29693.m002060 | 677801–676041 | - | Yes | - | - | IIc | 310 | 34.44 | 6.97 | -0.902 | AtWRKY28,08 | JcWRKY26 |
| RcWRKY24 | scaffold30076 | 1245583–1244284 | 30076.t000187 | 30076.m004623 | 1245777–1243970 | - | Yes | - | - | IIc | 317 | 35.19 | 6.40 | -0.619 | AtWRKY23 | JcWRKY22 |
| RcWRKY25 | scaffold30174 | 3224608–3221434 | 30174.t000532 | 30174.m009135 | 3225599–3218630 | 1 | Yes | - | Yes | IIc, misannotated | 308 | 33.78 | 6.19 | -0.909 | AtWRKY57 | JcWRKY24 |
| RcWRKY26 | scaffold29767 | 166469–163654 | 29767.t000010 | 29767.m000208 | 166564–163614 | - | Yes | - | - | IIc | 296 | 32.89 | 5.50 | -0.700 | AtWRKY49 | JcWRKY38 |
| RcWRKY27 | scaffold28842 | N/A | N/A | N/A | 266259–272883 | - | Yes | - | Yes | IIc, not predicted | 117 | 13.57 | 6.30 | -1.262 | - | JcWRKY58 |
| RcWRKY28 | scaffold30131 | 4672–6497 | 30131.t000001 | 30131.m006850 | 4396–6722 | 7 | Yes | - | Yes | IIa, misassembled | 318 | 35.49 | 8.64 | -0.803 | AtWRKY40,18,60 | JcWRKY27 |
| scaffold43951 | 916–31 | 43951.t000001 | 43951.m000016 | |||||||||||||
| RcWRKY29 | scaffold29848 | 538602–537211 | 29848.t000101 | 29848.m004545 | 538811–533980 | 1 | Yes | - | Yes | IIa, misannotated | 330 | 36.53 | 8.52 | -0.629 | AtWRKY40,18,60 | JcWRKY28 |
| RcWRKY30 | scaffold29848 | 523071–522197 | 29848.t000100 | 29848.m004544 | 523248–521822 | - | Yes | - | Yes | IIa, misannotated | 242 | 27.84 | 9.63 | -0.719 | AtWRKY40,18,60 | JcWRKY29 |
| RcWRKY31 | scaffold29842 | 273087–275527 | 29842.t000052 | 29842.m003555 | 272719–275658 | 1 | Yes | Yes | Yes | IIb | 580 | 63.10 | 5.95 | -0.686 | AtWRKY42,06,31 | JcWRKY31 |
| RcWRKY32 | scaffold30010 | 386731–384306 | 30010.t000025 | 30010.m000675 | 386795–384065 | 2 | Yes | - | - | IIb | 652 | 70.28 | 5.89 | -0.735 | AtWRKY42,06,31 | JcWRKY32 |
| RcWRKY33 | scaffold30076 | 678694–681282 | 30076.t000112 | 30076.m004548 | 678304–681837 | 1 | Yes | Yes | Yes | IIb | 498 | 53.86 | 8.01 | -0.545 | AtWRKY47 | JcWRKY30 |
| RcWRKY34 | scaffold30064 | 205783–203340 | 30064.t000028 | 30064.m000506 | 205918–203262 | - | Yes | - | - | IIb | 559 | 61.32 | 6.42 | -0.611 | AtWRKY47 | JcWRKY33 |
| RcWRKY35 | scaffold29736 | 182805–186919 | 29736.t000019 | 29736.m002023 | 182186–187534 | - | Yes | - | Yes | IIb, misannotated | 634 | 69.06 | 7.58 | -0.837 | AtWRKY72,61 | JcWRKY35 |
| RcWRKY36 | scaffold29822 | 915514–919280 | 29822.t000159 | 29822.m003484 | 915472–919280 | 6 | Yes | - | - | IIb | 560 | 60.07 | 6.51 | -0.606 | AtWRKY72,61 | JcWRKY37 |
| RcWRKY37 | scaffold30147 | 4428301–4432024 | 30147.t000358 | 30147.m014087 | 4428301–4432024 | - | Yes | - | - | IIb | 651 | 70.62 | 6.23 | -0.765 | AtWRKY72,61 | JcWRKY36 |
| RcWRKY38 | scaffold29990 | 4397–6433 | 29990.t000001 | 29990.m000497 | 4081–6735 | - | Yes | - | - | IIb | 532 | 58.99 | 5.25 | -0.814 | AtWRKY09 | JcWRKY34 |
| RcWRKY39 | scaffold29848 | 96608–94812 | 29848.t000020 | 29848.m004464 | 96843–94777 | 46 | Yes | - | Yes | IId | 321 | 34.61 | 9.54 | -0.485 | AtWRKY11,17 | JcWRKY43 |
| RcWRKY40 | scaffold29598 | 24172–22752 | 29598.t000004 | 29598.m000445 | 24740–22377 | - | Yes | - | Yes | IId, misannotated | 353 | 39.92 | 9.75 | -0.663 | AtWRKY74,39 | JcWRKY40 |
| RcWRKY41 | scaffold29644 | 80028–79578 | 29644.t000015 | 29644.m000187 | 81125–78861 | 45 | Yes | Yes | Yes | IId, misassembled | 356 | 38.95 | 9.43 | -0.630 | AtWRKY07 | JcWRKY41 |
| scaffold29644 | 81125–80117 | 29644.t000016 | 29644.m000188 | |||||||||||||
| RcWRKY42 | scaffold29883 | 450119–452078 | 29883.t000063 | 29883.m002008 | 449401–452648 | - | Yes | - | Yes | IId | 353 | 39.75 | 9.66 | -0.867 | AtWRKY21,39,74 | JcWRKY39 |
| RcWRKY43 | scaffold30170 | 1224146–1225662 | 30170.t000244 | 30170.m013832 | 1224113–1226229 | 1 | Yes | - | - | IId | 377 | 41.43 | 9.59 | -0.773 | AtWRKY07,15 | JcWRKY42 |
| RcWRKY44 | scaffold28455 | 136512–134590 | 28455.t000009 | 28455.m000362 | 137020–134740 | - | Yes | - | - | IId, misannotated | 317 | 35.36 | 9.76 | -0.584 | AtWRKY21 | JcWRKY44 |
| RcWRKY45 | scaffold30174 | 2058419–2060079 | 30174.t000060 | 30174.m008663 | 2058419–2060093 | 9 | Yes | Yes | Yes | IIe | 347 | 37.77 | 5.92 | -0.676 | AtWRKY22 | JcWRKY47 |
| RcWRKY46 | scaffold30190 | 3016527–3017971 | 30190.t000512 | 30190.m011276 | 3016527–3018091 | - | Yes | - | - | IIe | 334 | 37.45 | 4.98 | -0.601 | AtWRKY29 | JcWRKY45 |
| RcWRKY47 | scaffold30169 | 2000467–1998907 | 30169.t000358 | 30169.m006581 | 2000700–1998672 | - | Yes | - | - | IIe | 459 | 50.16 | 5.69 | -0.814 | AtWRKY27 | JcWRKY48 |
| RcWRKY48 | scaffold30076 | 1384793–1383612 | 30076.t000212 | 30076.m004648 | 1385658–1383503 | - | Yes | - | Yes | IIe | 265 | 30.19 | 5.33 | -0.988 | AtWRKY69,69 | JcWRKY46 |
| RcWRKY49 | scaffold30026 | 178343–179952 | 30026.t000025 | 30026.m001461 | 178328–180279 | 2 | Yes | - | - | IIe | 267 | 30.02 | 5.90 | -1.064 | AtWRKY69,69 | JcWRKY50 |
| RcWRKY50 | scaffold27996 | 7134–9912 | 27996.t000002 | 27996.m000145 | 6705–10469 | - | Yes | - | Yes | IIe, misannotated | 480 | 52.06 | 5.28 | -0.774 | AtWRKY35,14 | JcWRKY49 |
| RcWRKY51 | scaffold30190 | 3508463–3507055 | 30190.t000050 | 30190.m010814 | 3508665–3505132 | 2 | Yes | Yes | Yes | III | 338 | 38.01 | 5.41 | -0.831 | AtWRKY41,53 | JcWRKY54 |
| RcWRKY52 | scaffold30174 | 1952662–1954347 | 30174.t000048 | 30174.m008651 | 1952428–1954708 | - | Yes | - | - | III | 333 | 37.80 | 5.38 | -0.786 | AtWRKY30 | JcWRKY51 |
| RcWRKY53 | scaffold30169 | 1742567–1744656 | 30169.t000310 | 30169.m006533 | 1952662–1954347 | - | Yes | - | Yes | III | 370 | 41.17 | 5.54 | -0.697 | AtWRKY41,53 | JcWRKY53 |
| RcWRKY54 | scaffold28690 | 13680–12224 | 28690.t000001 | 28690.m000025 | 1742258–1744988 | - | Yes | - | - | III, misannotated | 339 | 38.05 | 5.56 | -0.663 | AtWRKY41,53 | JcWRKY52 |
| RcWRKY55 | scaffold29729 | 344149–342169 | 29729.t000063 | 29729.m002330 | 13973–11973 | - | Yes | - | - | III | 318 | 35.55 | 5.94 | -0.704 | AtWRKY55 | JcWRKY55 |
| RcWRKY56 | scaffold29729 | 570667–568893 | 29729.t000103 | 29729.m002370 | 344520–341549 | - | Yes | - | - | III | 331 | 36.91 | 5.94 | -0.693 | AtWRKY55 | JcWRKY55 |
| RcWRKY57 | scaffold29729 | 563671–564936 | 29729.t000102 | 29729.m002369 | 570667–568714 | 1 | Yes | - | Yes | III | 314 | 35.45 | 5.87 | -0.603 | AtWRKY70 | JcWRKY57 |
| RcWRKY58 | scaffold29915 | 143043–141304 | 29915.t000015 | 29915.m000479 | 563637–565065 | - | Yes | Yes | Yes | III | 330 | 37.07 | 5.53 | -0.667 | AtWRKY70 | JcWRKY56 |
a Based on the EST data.
b Based on the RNA sequencing data.
N/A, not available.
“-”, not detected.
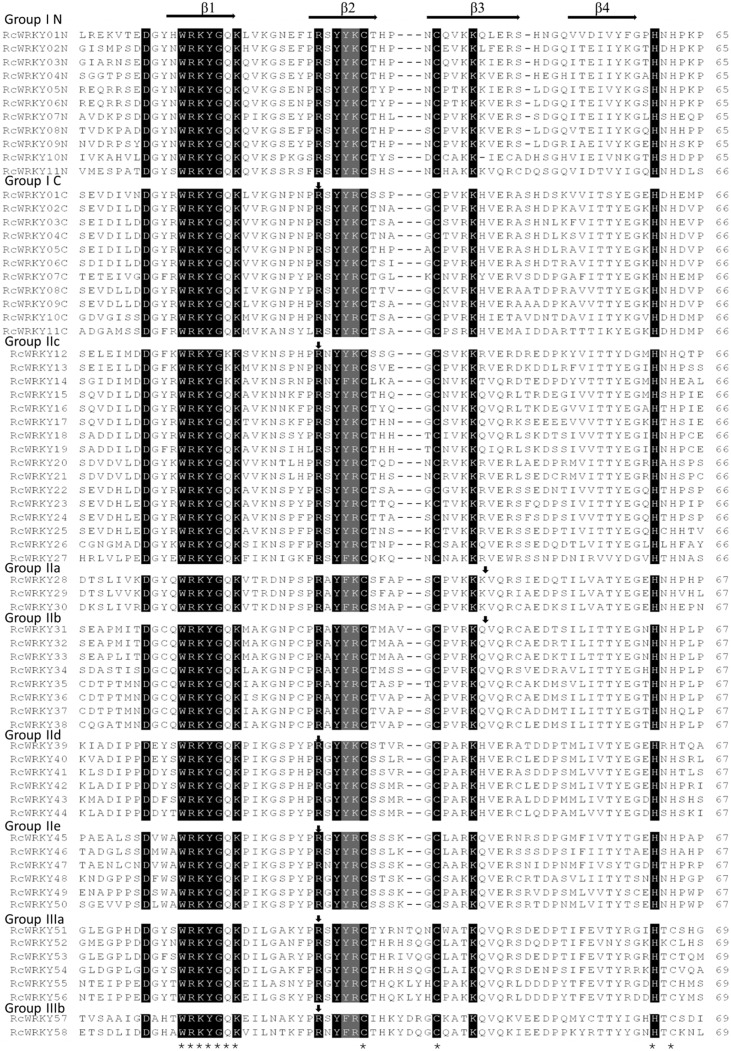
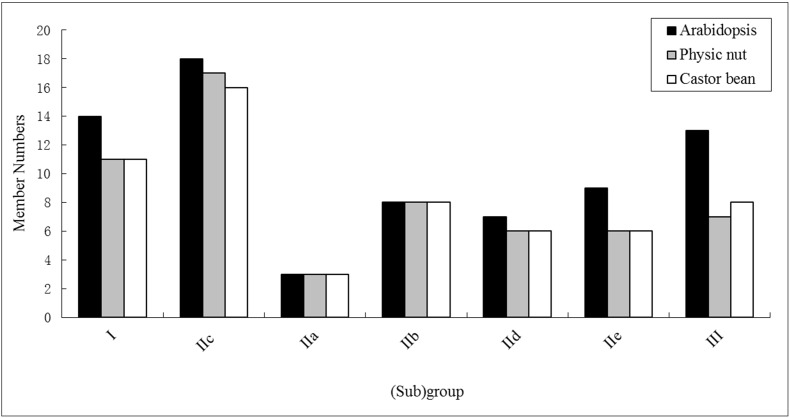
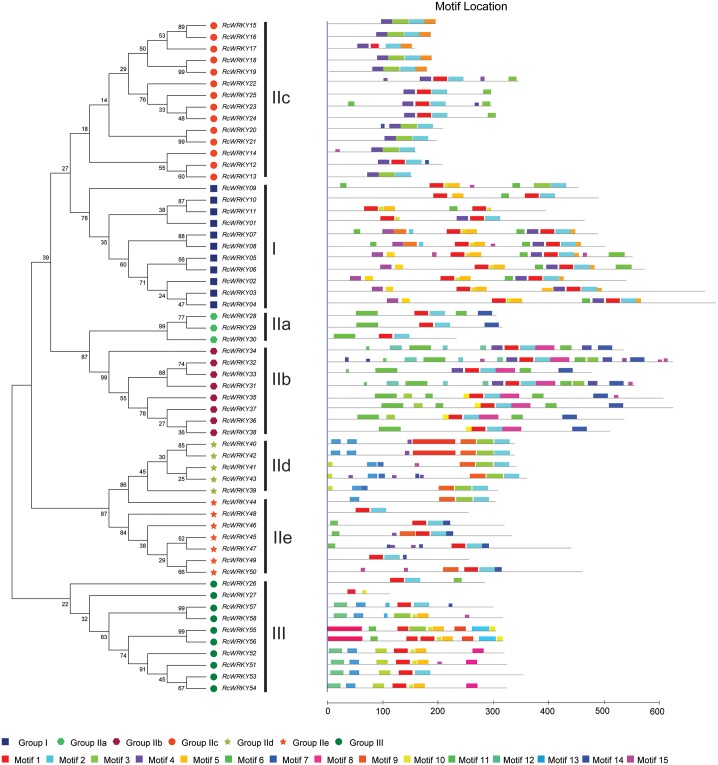
Based on the structural features (Fig 1) and evolutionary relationships (Fig 2, see below), a systematic name was assigned to each of the 58 RcWRKY genes (Table 1). Eleven members that contain two WRKY domains and feature the C2H2-type zinc finger motif (N: Cx4Cx22-23HxH; C: Cx4Cx23HxH) were categorized into the group I, whereas the remainings that harbor a single WRKY domain were categorized into the group II (39 members, featuring the C2H2 zinc finger: Cx4-5Cx23HxH) or III (8 members, featuring the C2HC zinc finger: Cx7Cx23HxC) (Table 1 and Fig 1). RcWRKY genes of the group II were further divided into 5 subgroups, i.e., IIa (3), IIb (8), IIc (16), IId (6) and IIe (6) (Fig 3). As shown in Fig 2, RcWRKY26 and RcWRKY27 seem to form two new subgroups: RcWRKY26, JcWRKY38 and AtWRKY49 were clustered together and shown to be closer to the N-terminal WRKY domains, whereas RcWRKY27 and its ortholog JcWRKY58 were closer to the group III members. However, both of them exhibit a zinc finger pattern Cx4Cx23HxH as observed in the subgroup IIc and the C-terminal WRKY domains of group I members (Fig 1). Thereby, they were classed into the subgroup IIc in this study. Compared with Arabidopsis, castor bean and physic nut have fewer family members in any (sub)group. Although the total number of family members is the same between castor bean and physic nut, castor bean contains one more group III member but one fewer subgroup IIc (Fig 3).
Fig 1. Comparison of the WRKY domain sequences from 58 RcWRKY proteins.
WRKY..N/C represents the N or C-terminal WRKY domain of group I members, respectively. “-” has been inserted for the optimal alignment. Conserved amino acid residues are shown in gray and the highly conserved WRKYGQ/KK heptapeptide and C2H2/C and residues are indicated by “*”. The four β-strands are indicated by right arrows. For each (sub)group, the position of a conserved intron is indicated by a down arrow.
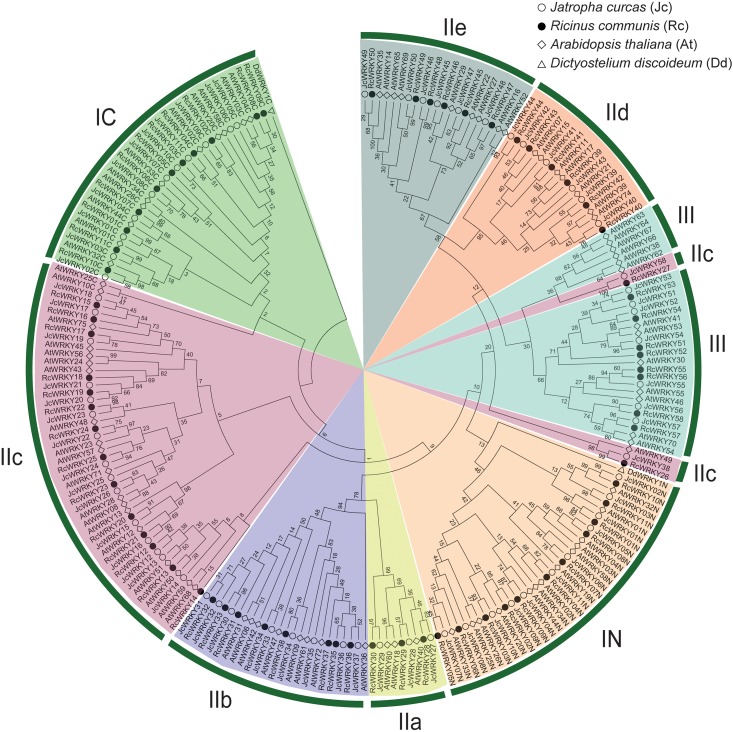
Fig 2. Phylogenetic analysis of RcWRKY proteins with Arabidopsis and physic nut homologs.
The WRKY domains (WRKY..N/C representing the N and C-termini of group I members, respectively) extracted from deduced amino acid sequences were performed using MUSCLE and the phylogenetic tree adopting DdWRKY1C as an outgroup was constructed using bootstrap maximum likelihood tree (1000 replicates) method and MEGA6 software. The distance scale denotes the number of amino acid substitutions per site. The name of each (sub)group is indicated next to the corresponding group. Species and accession numbers are listed in Table 1 and S1 Table.
Fig 3. Distribution of the 58 RcWRKY genes and their Arabidopsis and physic nut homologs in subgroups.
Although most RcWRKYs harbor the conserved heptapeptide WRKYGQK, the WRKYGKK variety was also observed in three members (i.e. RcWRKY12, RcWRKY13 and RcWRKY14) (Fig 1) as seen in physic nut, Arabidopsis and other plant species [7,10,12]. Except for the automatic genome annotation, homology analysis showed that no cDNA sequences of the 58 identified RcWRKY genes were reported in any public database. Nevertheless, 20 members had EST hits in NCBI GenBank (as of Apr 2015). Though most of them had only one hit, we still observed that three members (RcWRKY39, RcWRKY41 and RcWRKY05) matched more than 40 ESTs (Table 1). Further, read alignments against RNA sequencing data of root, leaf, flower, seed and endosperm supported the expression of other 38 RcWRKY genes. In addition, alternative splicing isoforms existing in 7 or 31 RcWRKY-encoding loci were supported by Sanger ESTs or RNA sequencing reads, respectively (Table 1).
As described above, the 1019-bp scaffold43951 was predicted to encode a WRKY domain-containing peptide. However, since it can be anchored to the 2696182-bp scaffold30131 sharing a 300-bp overlapping sequence, thus the scaffold30131 instead of scaffold43951 was counted as one WRKY-encoding scaffold. Among these 41 WRKY-encoding scaffolds, nine of them, i.e., scaffold30174 (5), scaffold29848 (4), scaffold30190 (4), scaffold30076 (3), scaffold29729 (3), scaffold29929 (2), scaffold30147 (2), scaffold29644 (2) and scaffold30169 (2), were shown to encode more than one WRKY genes, whereas the remainings encode a single one (Table 1).
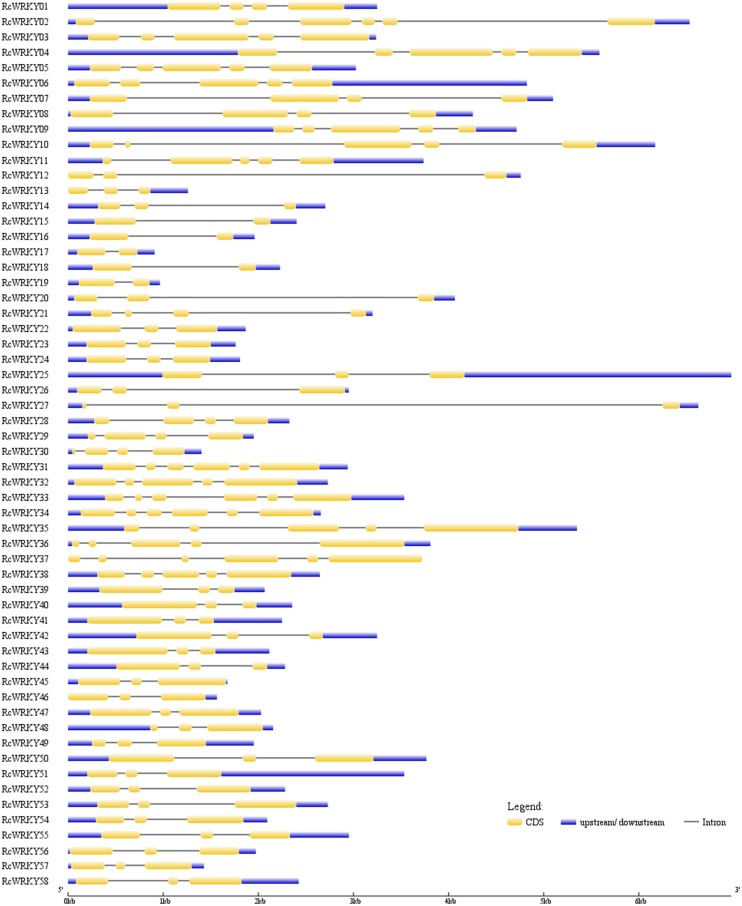
The exon-intron structures of the 58 RcWRKY genes were investigated based on the optimized gene models. Though all the deduced polypeptides of the RcWRKY genes contain one or two complete WRKY domains (Fig 1), the length of these amino acid sequences is highly distinct (Table 1). Compared with the CDS length (354–2202 bp), the gene length (from start to stop codons) of RcWRKYs is even more variable (633–6280 bp) (Fig 4). All RcWRKY genes contain at least one intron in their CDSs: 5 have one intron; 30 (more than 51.7%) have two introns, which include all members of (sub)groups IId, IIe and III; 7 have three introns; 11 have four introns; and 5 have five introns (Fig 4). Except for RcWRKY29, similar exon-intron structures were also observed in physic nut [7], a plant species also belonging to the Euphorbiaceae family and having diverged from castor bean approximately 49.4 million years ago [20]. Although the peptide length is very similar, RcWRKY29 (CDS, 993 bp) was shown to contain three introns (Fig 4); in contrast, its physic nut ortholog (JcWRKY28, CDS, 996 bp) has four introns [7]. Sequence analysis indicated that RcWRKY29 has lost the second intron as observed in physic nut. Without any exception, all RcWRKY genes harbor one intron in the WRKY domain-coding sequences (the C-terminal WRKY domain of group I members) (Fig 1). In members of subgroups a and b, the conserved intron presents in the zinc finger motif (24 codons further towards the C-terminus), whereas in groups I and III, and subgroups c–e, the intron is located after the second base of the arginine codon close to the N-termini of the zinc finger motif (Fig 1). Similar results were also observed in Arabidopsis and other plant species [6,10], suggesting that this is a general feature of the entire gene family.
Fig 4. Exon-intron structures of the 58 identified RcWRKY genes.
The graphic representation of the optimized gene models is displayed using GSDS.
Phylogenetic analysis of RcWRKY proteins
The homology analysis via Blastp showed that the 58 RcWRKYs have 56 or 36 counterparts in physic nut and Arabidopsis, respectively (Table 1), suggesting specific gene expansion and gene loss occurred in these plant species. Since the amino acid sequences beyond the WRKY domain are highly variable, the WRKY domain sequences were extracted from D. discoideum, Arabidopsis, physic nut and castor bean WRKY proteins, and used for the phylogenetic tree construction. D. discoideum, a slime mold closely related to the lineage of animals and fungi, was shown to encode a single group I-like WRKY gene which appears to be obtained via lateral gene transfer having occurred pre-date the formation of the WRKY groups in flowering plants [10,38]. The tree adopting DdWRKY1C as an outgroup was shown in Fig 2. According to the phylogenetic tree, a high number of Arabidopsis WRKY family members were grouped in pairs (Fig 2), corresponding to the occurrence of one whole-genome triplication event and two recent doubling events [39,40]. In contrast, few gene pairs were identified in castor bean as seen in physic nut (Fig 2). RcWRKY55 and RcWRKY56 were clustered together with their closest homolog in physic nut (JcWRKY55) (Fig 2). Both of them were clustered in scaffold29729 (spaced by 39 loci) (Table 1), indicating that they were resulted from proximal duplication after the divergence of castor bean and physic nut. In addition, the C-terminal WRKY domains of RcWRKY08 and RcWRKY09 were also clustered together apart from that of JcWRKY05 and JcWRKY06, however, the N-terminal WRKY domains of RcWRKY08 and RcWRKY09 were clustered with that of JcWRKY05 and JcWRKY04, respectively; moreover, the Blastp analysis indicated the ortholog of RcWRKY08 and RcWRKY09 is JcWRKY05 or JcWRKY04, respectively. Thereby, RcWRKY08 and RcWRKY09 are promised to emerge before the divergence of castor bean from physic nut. The homology analysis also suggested that the castor bean has lost the ortholog of JcWRKY25, since its ortholog was detected in another two Euphorbiaceae plants, i.e., cassava (Manihot esculenta) and rubber tree (Hevea brasiliensis) ([41,42] Zou et al., unpublished data).
Protein properties and conserved motifs beyond the WRKY domain
The predicted RcWRKY proteins have an average length of about 383 residues, with the minimum of 117 residues for RcWRKY27 and the maximum of 733 residues for RcWRKY04, whereas the average molecular weight is about 42.22 kDa, with the minimum of 13.57 kDa for RcWRKY27 and the maximum of 79.82 kDa for RcWRKY04, which is consistent with their peptide length. Although harboring an average pI value of 7.08, more than 58.62% RcWRKY proteins have a pI value of less than 7, indicating that most of them are acid. All RcWRKY proteins were predicted to harbor a GRAVY value (average: -0.78) of less than 0, indicating their hydrophilic feather. According to the 2ZIP analysis, two RcWRKY proteins (i.e. RcWRKY29 and RcWRKY33) were predicted to harbor a conserved Leu zipper motif, which was shown to be involved in dimerization and DNA binding [43,44]. The HARF motif was identified in three subgroup IId members, RcWRKY39, RcWRKY41 and RcWRKY43, although little is known about its exact function. LxxLL, a coactivator motif, was not found in any of the 58 RcWRKY proteins. In contrast, the active repressor motif LxLxLx were identified in two out of the three subgroup IIa members (i.e. RcWRKY28 and RcWRKY30) and four out of eight subgroup IIb members (i.e. RcWRKY35, RcWRKY36, RcWRKY37 and RcWRKY38).
To better understand the similarity and diversity of motif compositions among different RcWRKYs, a phylogenetic tree based on the full-length RcWRKY proteins was constructed (Fig 5) and the motifs in RcWRKY protein sequences were predicted using MEME (Fig 5, Table 2). Among 15 identified motifs, motifs 1, 2, 3 and 10 were characterized as WRKY domains that are broadly distributed across the RcWRKYs; the motif 9, characterized as the nuclear localization signal (NLS) sequence, was found in all members of subgroups IIa and IIb. In contrast, little information is available for other motifs: the motif 4 was found in most members of the group I, subgroups IIb and IIc; motifs 5 and 10 were found in most members of groups I and III; the motif 13 was found in the subgroup IId and group III; motifs 6, 7, 8 and 11 are limited to subgroups IIa and IIb members; motifs 12 and 15 are unique in the group III or I, respectively.
Fig 5. Structural and phylogenetic analysis of RcWRKY proteins.
The unrooted phylogenetic tree resulting from the full-length amino acid alignment of all the RcWRKY proteins is shown on the left side of the figure. The different colored balls at the bottom of the figure indicate different groups. The distribution of conserved motifs among the RcWRKY proteins is shown on the right side of the figure. Different motif types are represented by different color blocks as indicated at the bottom of the figure. The same color in different proteins indicates the same group or motif.
Table 2. Motif sequences of 58 RcWRKY proteins identified by the MEME tools.
| Motif | E-value | Sites | Width | Best possible match |
|---|---|---|---|---|
| Motif1 | 3.0E-1047 | 50 | 26 | DGYRWRKYGQKMVKGNPYPRSYYRCT |
| Motif2 | 5.2E-871 | 50 | 29 | GCPVRKHVERCAEDPTMVITTYEGEHNH |
| Motif3 | 9.7E-326 | 17 | 31 | DILDDGYRWRKYGQKPIKNSPHPRGYYRCTH |
| Motif4 | 8.80E-114 | 26 | 21 | KKKGEKKIREPRFAFQTRSEV |
| Motif5 | 8.80E-89 | 17 | 21 | ERDHDGQIFEIIYKGTHNCPK |
| Motif6 | 3.90E-95 | 11 | 41 | LEVLQAELERMKEENERLRQMLTQMCKNYNALQMHFCELMQ |
| Motif7 | 6.20E-78 | 10 | 27 | VEAATAAITADPNFTAALAAAITSIIG |
| Motif8 | 1.20E-72 | 8 | 36 | AAMAMASTTSAAASMLLSGSSSSADGIMNHNTF |
| Motif9 | 5.90E-58 | 8 | 29 | CASSGRCHCSKRRKMRVKRVIRVPAISNK |
| Motif10 | 4.20E-30 | 18 | 8 | NCPAKKKV |
| Motif11 | 3.40E-29 | 8 | 21 | MASISASAPFPTITLDLTHS |
| Motif12 | 3.00E-24 | 6 | 25 | WEQHTLVGELIQGRELARQLRIHLN |
| Motif13 | 1.10E-22 | 14 | 18 | LVQKIVSKFKKVLSLLNW |
| Motif14 | 1.80E-17 | 10 | 7 | NHHHHHH |
| Motif15 | 4.00E-20 | 7 | 21 | SPYITIPPGLSPTALLDSPVF |
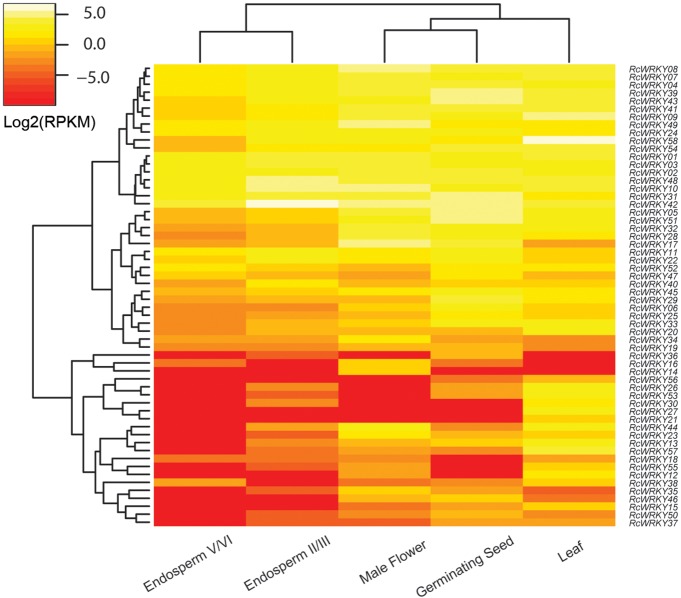
Distinct expression profiles of RcWRKY family members in various tissues
To gain more information on the role of WRKY genes in castor bean, RNA sequencing data of leaf, male flower, endosperm and seed were investigated. The expanding true leaves, appearing after the first cotyledons and leaf-pair, represent the leaf tissue; the male flower tissue includes pollen and anthers but excludes sepals; the germinating seed tissue was obtained by soaking dry seeds in running water overnight followed by germination in the dark for 3 days; and the endosperm tissue includes two representative stages termed stages II/III (endosperm free-nuclear stage) and V/VI (onset of cellular endosperm development) [24]. Results showed that the expression of all 58 RcWRKY genes were detected in at least one of the examined tissues, i.e., 55 in leaf, 51 in male flower, 51 in endosperm and 51 in seed (Fig 6). And the cluster analysis showed that the expression pattern of RcWRKY genes was more similar between flower and seed, and two stages of endosperm (Fig 6), corresponding their biological characteristics. Among three genes not detected in leaves, RcWRKY14 was only and lowly expressed in male flowers, although previous qRT-PCR analysis showed that it was also expressed in roots and fruits at 50 days post-anthesis [21]. In contrast, its ortholog JcWRKY14 in physic nut was shown to be highly expressed in stems (shoot cortex), roots and seeds of late development (i.e. filling and maturation) stage as well as leaves [8]. RcWRKY16 was expressed in male flowers, germinating seeds and stage V/VI endosperm, and the expression levels were considerably low in seeds and endosperm, which is consistent with the qRT-PCR result [21]. Similar expression profile of its ortholog JcWRKY18 in physic nut was also observed [8]. RcWRKY36 was detected in stage II/III endosperm and germinating seeds, and the previous qRT-PCR analysis indicated that this gene was highly expressed in roots [21]. In physic nut, the expression of its ortholog JcWRKY37 was also shown to be restricted to roots [8]. Among seven genes not detected in male flowers, all of them were also not detected in stage V/VI endosperm; except for RcWRKY36, RcWRKY21, RcWRKY26, RcWRKY27, RcWRKY30, RcWRKY53 and RcWRKY56 were all detected in leaves; RcWRKY21 and RcWRKY27 were detected only in leaves; besides leaves, RcWRKY26 and RcWRKY30 were also detected in stage II/III endosperm, though the expression level was extremely low; RcWRKY53 was also detected lowly in stage II/III endosperm and germinating seeds; and RcWRKY56 was also detected in germinating seeds. Among seven genes not detected in endosperm, RcWRKY14, RcWRKY21, RcWRKY27 and RcWRKY56 were discussed above; RcWRKY12 was detected in leaves and male flowers which is consistent with the qRT-PCR result [21] and the expression pattern of its ortholog JcWRKY12 in physic nut [8]; RcWRKY15 and RcWRKY56 were lowly expressed in all other samples examined, in contrast, their physic nut orthologs JcWRKY17 and JcWRKY45 were shown to be highly expressed in roots, lowly expressed in stems and leaves, but not detected seeds of both early and late development stages [8]. Among seven genes (i.e. RcWRKY12, RcWRKY14, RcWRKY18, RcWRKY21, RcWRKY27, RcWRKY30 and RcWRKY55) not detected in germinating seeds, RcWRKY12, RcWRKY14, RcWRKY21 and RcWRKY27 were discussed above. RcWRKY18 was lowly expressed in all other samples examined. Compared with other tissues [21], qRT-PCR analysis showed that RcWRKY18 was considerably more expressed in roots, which is consistent with the root-preferred expression of its ortholog JcWRKY21 in physic nut [8]. RcWRKY30 was only detected in leaves and stage II/III endosperm, whereas its physic nut ortholog JcWRKY29 was shown to be lowly expressed in leaves, stems and roots, but not seeds [8]. RcWRKY55 was lowly expressed in all other examined samples except for stage V/VI endosperm, in contrast, the expression of its physic nut ortholog JcWRKY55 was shown to be restricted to roots and the expression level was extremely low [8].
Fig 6. Expression profiles of the 58 RcWRKY genes in leaf, flower, endosperm II/III, endosperm V/VI and seed.
Color scale represents RPKM normalized log2 transformed counts and red indicates low expression and yellow indicates high expression.
Based on the RPKM annotation, the total transcript abundance of RcWRKY genes in endosperm tissue (including both stages II/III and V/VI, with RPKM = 337.14 or 123.03, respectively) was relatively lower than that in other three tissues, i.e., leaf (RPKM = 585.83), male flower (RPKM = 576.19) and germinating seed (RPKM = 560.44) (Fig 6). RcWRKY58 (RPKM = 139.35), the most abundant WRKY family member in leaves, was detected in all other tissues examined, though the expression levels were considerably low. Similarly, its ortholog AtWRKY70 in Arabidopsis was also shown to be constitutively expressed during all leaf development stages [45,46]. Functional analysis indicated that AtWRKY70 plays a pivotal role in salicylic acid (SA)- and jasmonic acid (JA)-dependent defense signaling [47,48]. Moreover, AtWRKY70 together with AtWRKY54 co-operate as negative regulators of leaf senescence and modulate osmotic stress tolerance by regulating stomatal movement [46,49,50]. Besides highly expressed in leaves, its ortholog JcWRKY56 in physic nut was even more abundant in seeds of early development stage, and the expression levels in roots, stems and leaves were up-regulated by stresses such as drought and salinity [8]. RcWRKY49 (RPKM = 49.08), the most expressed RcWRKY gene in male flowers, was also lowly detected in other tissues, which is consistent with the qRT-PCR result [21]. In contrast, its ortholog JcWRKY50 in physic nut was expressed highly in roots, moderately in leaves and lowly in stems, and the expression levels were regulated by at least one of tested abiotic stresses, i.e. drought, salinity, phosphate starvation and nitrogen starvation [8]. Among two highly abundant RcWRKY genes in germinating seeds, RcWRKY42 (RPKM = 49.46) also represented the most expressed member in stages II/III (RPKM = 81.26) and V/VI (RPKM = 21.86) endosperm, whereas RcWRKY05 (RPKM = 47.04) was expressed moderately in male flowers (RPKM = 19.96) and leaves (RPKM = 7.90), lowly in stages II/III (RPKM = 2.61) and V/VI (RPKM = 0.62) (Fig 6). Although not detected in two seed development stages, the physic nut ortholog (JcWRKY39) of RcWRKY42 was highly expressed in roots, leaves and stems, and the expression levels were regulated by nitrogen starvation [8]. The expression levels of the physic nut ortholog (JcWRKY07) of RcWRKY05 were shown to be high in roots, leaves and early developmental seeds, and extremely low in stems [8]. The response of JcWRKY07 to drought, salinity and phosphate starvation stresses was observed in roots [8]. AtWRKY33, an Arabidopsis ortholog of RcWRKY05 was shown to function as a positive regulator of resistance toward the necrotrophic fungi Alternaria brassicicola and Botrytis cinerea [51,52], and gene overexpression can increases salt and heat tolerance [53,54].
As mentioned above, the total RcWRKY transcripts in stage II/III endosperm was two folds more than that in stage V/VI endosperm. Among 51 RcWRKY genes detected in endosperm, 34 members had a RPKM value exceeding 0.5 in at least one stage of developing endosperm (stages II/III and V/VI). Differential expression analysis indicated that 23 out of the 32 down-regulated RcWRKY genes and one out of two up-regulated genes exceeded two folds (Fig 6), suggesting their putative regulatory role in early endosperm development.
In addition, RcWRKY genes are promised to be involved in the ABA-mediated seed filling. In vivo experiment showed that endogenous ABA levels were closely associated with storage material accumulation in developing castor bean seeds [55]. In vitro, exogenous ABA also enhanced the dry weight (including the accumulation of soluble sugar and total lipid content) of developing seeds cultured in a nutrient medium [56]. After the application of 10 μM ABA for 24 h, differential gene expression analysis indicated that 2568 genes were up or down-regulated at least two folds [56], which was shown to include 13 out of the 58 RcWRKY genes (S21 File). Among them, eleven (four group I members, two subgroup IId members, one subgroup IIa member, one subgroup IIb member, one subgroup IIc member, one subgroup IIe member and one group III member) were significantly up-regulated, whereas only two (one subgroup IIe member and one group III member) were down-regulated. RcWRKY41, the most up-regulated gene (more than 250 folds) (S21 File), was highly expressed in germinating seeds, leaves and male flowers (Fig 6), which is consistent with its high representative in Genbank EST database (Table 1); its ortholog AtWRKY11 in Arabidopsis, was also shown to be constitutively expressed and act as negative regulators of basal resistance to Pseudomonas syringae [57]. RcWRKY28, the second highly up-regulated gene (more than 15 folds) (S21 File), was expressed more in male flowers and germinating seeds than in leaves and endosperm, though its expression level was considerably lower in stage V/VI endosperm as compared with stage II/III (Fig 6); AtWRKY40, its ortholog in Arabidopsis, was also induced by ABA and acts as a transcriptional repressor in ABA signaling and abiotic stress but a positive regulator in effector-triggered immunity [58–63]. RcWRKY17, a group IIc member preferring to express in male flowers, female flowers and germinating seeds, was up-regulated for more than nine folds upon the ABA application; its ortholog AtWRKY75 in Arabidopsis, was shown to response to phosphate starvation, water deprivation, ethylene stimulus and biotic stress, and participate in lateral root development, leaf senescence and galactolipid biosynthesis [64–67]. RcWRKY45, a group IIe member preferring to express in germinating seeds and fruits at 50 days post-anthesis [21], was up-regulated for more than seven folds by ABA; AtWRKY22, its ortholog in Arabidopsis, was involved in dark-induced leaf senescence and submergence-mediated immunity [68–69]. These results suggested the putative role of RcWRKYs in the ABA signaling.
Conclusions
Based on the genome and transcriptome datasets, in the current study, a total of 58 WRKY genes were identified from castor bean, one of the most important non-food oilseed crops in the Euphorbiaceae family. According to the structural features and evolutionary relationships of the present WRKY domains, the identified RcWRKY genes were assigned to the group I, group II (subgroup a-e) and group III. The WRKY domain pattern was characterized as WRKYGQ/KKx13Cx4-7Cx22-23HxH/C. Compared with Arabidopsis that feathers a high number of duplicate genes, few gene pairs were identified in the RcWRKY gene family, corresponding to no recent whole-genome duplication event occurred in castor bean. Comparative genomics analysis also indicated that one gene loss, one intron loss and one recent proximal duplication occurred in the RcWRKY gene family as compared with physic nut, another Euphorbiaceae plant species underwent no recent whole-genome duplication event. Although only 20 family members had EST hits in public database, the expression of all 58 RcWRKY genes was supported by RNA sequencing reads derived from root, leaf, flower, seed and endosperm. Compared with tissues such as leaf, male flower and germinating seed, the total expression level of RcWRKY genes in endosperm tissue was shown to be relatively low. Distinct gene expression profiles were also observed in different developmental endosperm. Compared with stage II/III endosperm, 23 out of the 54 endosperm-expressed RcWRKY genes were down-regulated at least two folds at stage V/VI, whereas only one member was shown to be significantly up-regulated, suggesting their key regulatory role in early endosperm development. In a word, results obtained from this study not only provide global information in understanding the molecular basis of the WRKY gene family in castor bean, but also provide a useful reference to investigate the gene family expansion and evolution in Euphorbiaceus plants such as Hevea brasiliensis and Manihot esculenta, and other plant species that underwent recent whole-genome duplication events.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(XLSX)
Acknowledgments
The authors appreciate those contributors who make the castor bean genome and transcriptome data accessible in public databases.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The Funding should be the National Natural Science Foundation of China (31371556), the Fundamental Research Funds for Rubber Research Institute, CATAS (1630022015004) and the Earmarked Fund for Modern Agro-industry Technology Research System (CARS-34-GW5).
References
- 1.Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends Plant Sci. 2010; 15(5): 247–258. 10.1016/j.tplants.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Song Y, Li S, Zhang L, Zou C, Yu D. The role of WRKY transcription factors in plant abiotic stresses. Biochim Biophys Acta. 2012; 1819(2): 120–128. 10.1016/j.bbagrm.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 3.Schluttenhofer C, Yuan L. Regulation of specialized metabolism by WRKY transcription factors. Plant Physiol. 2015; 167(2): 295–306. 10.1104/pp.114.251769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishiguro S, Nakamura K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5' upstream regions of genes coding for sporamin and beta-amylase from sweet potato. Mol Gen Genet. 1994; 244(6): 563–571. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Wang L. The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol Biol. 2005; 5: 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinerson CI, Rabara RC, Tripathi P, Shen QJ, Rushton PJ. The evolution of WRKY transcription factors. BMC Plant Biol. 2015; 15: 66 10.1186/s12870-015-0456-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling J, Jiang W, Zhang Y, Yu H, Mao Z, Gu X, et al. Genome-wide analysis of WRKY gene family in Cucumis sativus. BMC Genomics. 2011; 12: 471 10.1186/1471-2164-12-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong W, Xu X, Zhang L, Wu P, Chen Y, Li M, et al. Genome-wide analysis of the WRKY gene family in physic nut (Jatropha curcas L.). Gene. 2013; 524(2): 124–132. 10.1016/j.gene.2013.04.047 [DOI] [PubMed] [Google Scholar]
- 9.Guo C, Guo R, Xu X, Gao M, Li X, Song J, et al. Evolution and expression analysis of the grape (Vitis vinifera L.) WRKY gene family. J Exp Bot. 2014; 65(6): 1513–1528. 10.1093/jxb/eru007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000; 5(5): 199–206. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Li K, Xu X, Yao Z, Jin C, Zhang S. Genome-wide analysis of WRKY transcription factors in white pear (Pyrus bretschneideri) reveals evolution and patterns under drought stress. BMC Genomics. 2015; 16(1): 1104 10.1186/s12864-015-2233-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He H, Dong Q, Shao Y, Jiang H, Zhu S, Cheng B, et al. Genome-wide survey and characterization of the WRKY gene family in Populus trichocarpa. Plant Cell Rep. 2012; 31(7): 1199–1217. 10.1007/s00299-012-1241-0 [DOI] [PubMed] [Google Scholar]
- 13.Muthamilarasan M, Bonthala VS, Khandelwal R, Jaishankar J, Shweta S, Nawaz K, et al. Global analysis of WRKY transcription factor superfamily in Setaria identifies potential candidates involved in abiotic stress signaling. Front Plant Sci. 2015; 6: 910 10.3389/fpls.2015.00910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross CA, Liu Y, Shen QJ. The WRKY gene family in rice (Oryza sativa). J Integr Plant Biol. 2007; 49: 827–842. [Google Scholar]
- 15.Rushton PJ, Macdonald H, Huttly AK, Lazarus CM, Hooley R. Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of alpha-Amy2 genes. Plant Mol Biol. 1995; 29(4): 691–702. [DOI] [PubMed] [Google Scholar]
- 16.Yamasaki K, Kigawa T, Watanabe S, Inoue M, Yamasaki T, Seki M, et al. Structural basis for sequence-specific DNA recognition by an Arabidopsis WRKY transcription factor. J Biol Chem. 2012; 287(10): 7683–7691. 10.1074/jbc.M111.279844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou Z. Genome-wide identification and phylogenetic analysis of WRKY transcription factor family in Ricinus communis L. Chin J Oil Crop Sci. 2013; 35(1): 36–42. [Google Scholar]
- 18.Qiu L, Yang C, Tian B, Yang JB, Liu A. Exploiting EST databases for the development and characterization of EST-SSR markers in castor bean (Ricinus communis L.). BMC Plant Biol. 2010; 10: 278 10.1186/1471-2229-10-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivarola M, Foster JT, Chan AP, Williams AL, Rice DW, Liu X, et al. Castor bean organelle genome sequencing and worldwide genetic diversity analysis. PLoS One. 2011; 6(7): e21743 10.1371/journal.pone.0021743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan AP, Crabtree J, Zhao Q, Lorenzi H, Orvis J, Puiu D, et al. Draft genome sequence of the oilseed species Ricinus communis. Nat Biotechnol. 2010; 28(9): 951–956. 10.1038/nbt.1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li HL, Zhang LB, Guo D, Li CZ, Peng SQ. Identification and expression profiles of the WRKY transcription factor family in Ricinus communis. Gene. 2012; 503(2): 248–253. 10.1016/j.gene.2012.04.069 [DOI] [PubMed] [Google Scholar]
- 22.Wu P, Zhou C, Cheng S, Wu Z, Lu W, Han J, et al. Integrated genome sequence and linkage map of physic nut (Jatropha curcas L.), a biodiesel plant. Plant J. 2015; 81(5): 810–821. 10.1111/tpj.12761 [DOI] [PubMed] [Google Scholar]
- 23.Troncoso-Ponce MA, Kilaru A, Cao X, Durrett TP, Fan J, Jensen JK, et al. Comparative deep transcriptional profiling of four developing oilseeds. Plant J. 2011; 68(6): 1014–1027. 10.1111/j.1365-313X.2011.04751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown AP, Kroon JT, Swarbreck D, Febrer M, Larson TR, Graham IA, et al. Tissue-specific whole transcriptome sequencing in castor, directed at understanding triacylglycerol lipid biosynthetic pathways. PLoS One. 2012; 7(2): e30100 10.1371/journal.pone.0030100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu W, Li F, Ling L, Liu A. Genome-wide survey and expression profiles of the AP2/ERF family in castor bean (Ricinus communis L.). BMC Genomics. 2013; 14: 785 10.1186/1471-2164-14-785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu W, Dai M, Li F, Liu A. Genomic imprinting, methylation and parent-of-origin effects in reciprocal hybrid endosperm of castor bean. Nucleic Acids Res. 2014; 42(11): 6987–6998. 10.1093/nar/gku375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997; 25(17): 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonnhammer EL, Eddy SR, Birney E, Bateman A, Durbin R. Pfam: Multiple sequence alignments and HMM-profiles of protein domains. Nucleic Acids Res. 1998; 26: 320–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Letunic I, Doerks T, Bork P. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res. 2015; 43(Database issue): D257–260. 10.1093/nar/gku949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu B, Jin J, Guo A, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015; 31(8): 1296–1297. 10.1093/bioinformatics/btu817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012; 9(4): 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012; 7(3): 562–578. 10.1038/nprot.2012.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004; 32(5): 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013; 30(12): 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009; 37(Web Server issue): W202–208. 10.1093/nar/gkp335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat Methods. 2008; 5(7): 621–628. 10.1038/nmeth.1226 [DOI] [PubMed] [Google Scholar]
- 37.Jin JP, Zhang H, Kong L, Gao G, Luo JC. PlantTFDB 3.0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res. 2014; 42(Database issue): D1182–1187. 10.1093/nar/gkt1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glöckner G, Eichinger L, Szafranski K, Pachebat JA, Bankier AT, Dear PH, et al. Sequence and analysis of chromosome 2 of Dictyostelium discoideum. Nature. 2002; 418(6893): 79–85. [DOI] [PubMed] [Google Scholar]
- 39.Bowers JE, Chapman BA, Rong J, Paterson AH. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature. 2003; 422(6930): 433–438. [DOI] [PubMed] [Google Scholar]
- 40.Blanc G, Wolfe KH. Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. Plant Cell. 2004; 16(7): 1679–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahman AY, Usharraj AO, Misra BB, Thottathil GP, Jayasekaran K, Feng Y, et al. Draft genome sequence of the rubber tree Hevea brasiliensis. BMC Genomics. 2013; 14: 75 10.1186/1471-2164-14-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prochnik S, Marri PR, Desany B, Rabinowicz PD, Kodira C, Mohiuddin M, et al. The cassava genome: current progress, future directions. Trop Plant Biol. 2012; 5(1): 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988; 240(4860): 1759–1764. [DOI] [PubMed] [Google Scholar]
- 44.Robatzek S, Somssich IE. A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence-related processes. Plant J. 2001; 28(2): 123–133. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004; 136(1): 2621–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulker B, Shahid Mukhtar M, Somssich IE. The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta. 2007; 226: 125–137. [DOI] [PubMed] [Google Scholar]
- 47.Li J, Brader G, Palva ET. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell. 2004; 16: 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Brader G, Kariola T, Palva ET. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006; 46: 477–491. [DOI] [PubMed] [Google Scholar]
- 49.Besseau S, Li J, Palva ET. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J Exp Bot. 2012; 63(7): 2667–2679. 10.1093/jxb/err450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Besseau S, Törönen P, Sipari N, Kollist H, Holm L, et al. Defense-related transcription factors WRKY70 and WRKY54 modulate osmotic stress tolerance by regulating stomatal aperture in Arabidopsis. New Phytol. 2013; 200(2): 457–472. 10.1111/nph.12378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Z, Qamar SA, Chen Z, Mengiste T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006; 48(4): 592–605. [DOI] [PubMed] [Google Scholar]
- 52.Birkenbihl RP, Diezel C, Somssich IE. Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol. 2012; 159(1): 266–285. 10.1104/pp.111.192641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Y, Deyholos MK. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol Biol. 2009; 69(1–2): 91–105. 10.1007/s11103-008-9408-3 [DOI] [PubMed] [Google Scholar]
- 54.Li S, Fu Q, Chen L, Huang W, Yu D. Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta. 2011; 233(6): 1237–52. 10.1007/s00425-011-1375-2 [DOI] [PubMed] [Google Scholar]
- 55.Chandrasekaran U, Liu A. Endogenous abscisic acid signaling towards storage reserve filling in developing seed tissues of castor bean (Ricinus communis L.). Plant Growth Regul. 2014; 72: 203–207. [Google Scholar]
- 56.Chandrasekaran U, Xu W, Liu A. Transcriptome profiling identifies ABA mediated regulatory changes towards storage filling in developing seeds of castor bean (Ricinus communis L.). Cell Biosci. 2014; 4: 33 10.1186/2045-3701-4-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Journot-Catalino N, Somssich IE, Roby D, Kroj T. The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell. 2006; 18(11): 3289–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen H, Lai Z, Shi J, Xiao Y, Chen Z, Xu X. Roles of arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol. 2010; 10: 281 10.1186/1471-2229-10-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shang Y, Yan L, Liu ZQ, Cao Z, Mei C, Xin Q, et al. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell. 2010; 22(6): 1909–1935. 10.1105/tpc.110.073874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pandey SP, Roccaro M, Schön M, Logemann E, Somssich IE. Transcriptional reprogramming regulated by WRKY18 and WRKY40 facilitates powdery mildew infection of Arabidopsis. Plant J. 2010; 64(6): 912–923. 10.1111/j.1365-313X.2010.04387.x [DOI] [PubMed] [Google Scholar]
- 61.Schön M, Töller A, Diezel C, Roth C, Westphal L, Wiermer M, et al. Analyses of wrky18 wrky40 plants reveal critical roles of SA/EDS1 signaling and indole-glucosinolate biosynthesis for Golovinomyces orontii resistance and a loss-of resistance towards Pseudomonas syringae pv. tomato AvrRPS4. Mol Plant Microbe Interact. 2013; 26(7): 758–767. 10.1094/MPMI-11-12-0265-R [DOI] [PubMed] [Google Scholar]
- 62.Van Aken O, Zhang B, Law S, Narsai R, Whelan J. AtWRKY40 and AtWRKY63 modulate the expression of stress-responsive nuclear genes encoding mitochondrial and chloroplast proteins. Plant Physiol. 2013; 162(1): 254–271. 10.1104/pp.113.215996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu R, Xu YH, Jiang SC, Lu K, Lu YF, Feng XJ, et al. Light-harvesting chlorophyll a/b-binding proteins, positively involved in abscisic acid signalling, require a transcription repressor, WRKY40, to balance their function. J Exp Bot. 2013; 64(18): 5443–5456. 10.1093/jxb/ert307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Devaiah BN, Karthikeyan AS, Raghothama KG. WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol. 2007;143(4): 1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Encinas-Villarejo S, Maldonado AM, Amil-Ruiz F, de los Santos B, Romero F, Pliego-Alfaro F, et al. Evidence for a positive regulatory role of strawberry (Fragaria x ananassa) FaWRKY1 and Arabidopsis AtWRKY75 proteins in resistance. J Exp Bot. 2009; 60(11): 3043–3065. 10.1093/jxb/erp152 [DOI] [PubMed] [Google Scholar]
- 66.Li Z, Peng J, Wen X, Guo H. Gene network analysis and functional studies of senescence-associated genes reveal novel regulators of Arabidopsis leaf senescence. J Integr Plant Biol. 2012; 54(8): 526–539. 10.1111/j.1744-7909.2012.01136.x [DOI] [PubMed] [Google Scholar]
- 67.Chen X, Liu J, Lin G, Wang A, Wang Z, Lu G. Overexpression of AtWRKY28 and AtWRKY75 in Arabidopsis enhances resistance to oxalic acid and Sclerotinia sclerotiorum. Plant Cell Rep. 2013; 32(10): 1589–1599. 10.1007/s00299-013-1469-3 [DOI] [PubMed] [Google Scholar]
- 68.Zhou X, Jiang Y, Yu D. WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis. Mol Cells. 2011; 31(4): 303–313. 10.1007/s10059-011-0047-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsu FC, Chou MY, Chou SJ, Li YR, Peng HP, Shih MC. Submergence confers immunity mediated by the WRKY22 transcription factor in Arabidopsis. Plant Cell. 2013; 25(7): 2699–2713. 10.1105/tpc.113.114447 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.